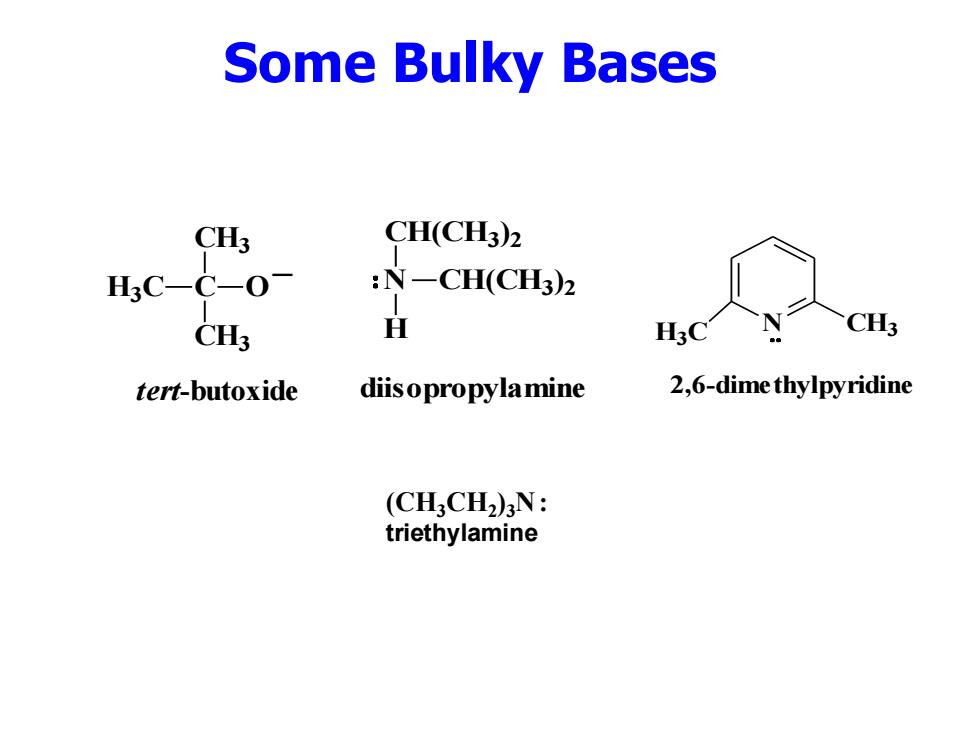
Some Bulky Bases CH3 CH(CH3)2 H3C-C-0 :N一CH(CH3)2 CH3 H CH3 tert-butoxide diisopropylamine 2,6-dime thylpyridine (CHCH2)N: triethylamine
Some Bulky Bases C CH3 H3C CH3 O _ tert-butoxide (CH3CH2 )3N : triethylamine N H CH(CH3 ) 2 CH(CH3 ) 2 diisopropylamine H N CH3 3C 2,6-dimethylpyridine
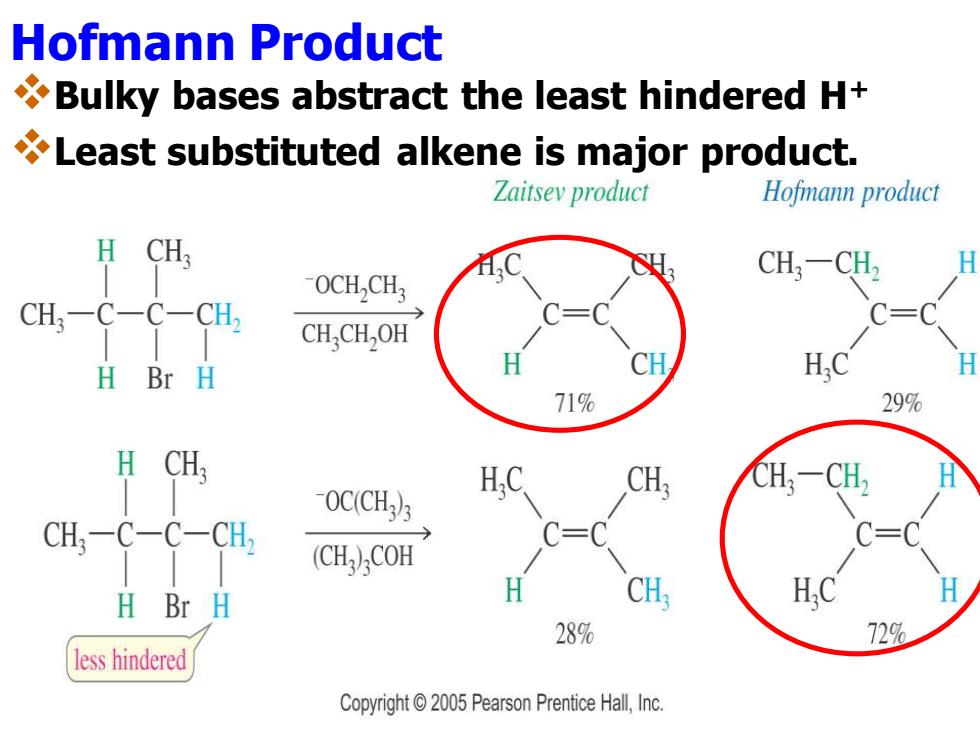
Hofmann Product Bulky bases abstract the least hindered H+ Least substituted alkene is major product. Zaitsey product Hofmann product H CH OCH,CH3 CH:-CH2 CH;-C-C- CH, CH,CH,OH H Br H HC H 71% 29% -0CCH为 CH; CH;-C-C-CH, (CH)COH H Br H CH, 289% 729% less hindered Copyright 2005 Pearson Prentice Hall,Inc
Hofmann Product ❖Bulky bases abstract the least hindered H+ ❖Least substituted alkene is major product
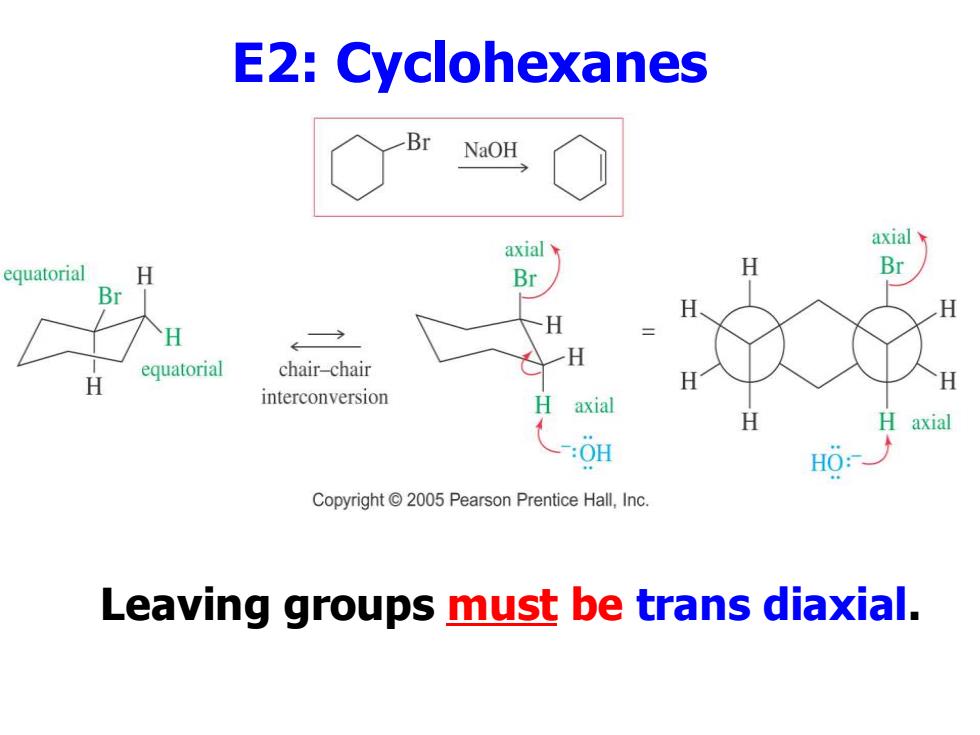
E2:Cyclohexanes NaOH axial axial equatorial H Br H Br Br H H equatorial H chair-chair H H H interconversion axial H H axial :0H H0: Copyright 2005 Pearson Prentice Hall,Inc. Leaving groups must be trans diaxial
E2: Cyclohexanes Leaving groups must be trans diaxial
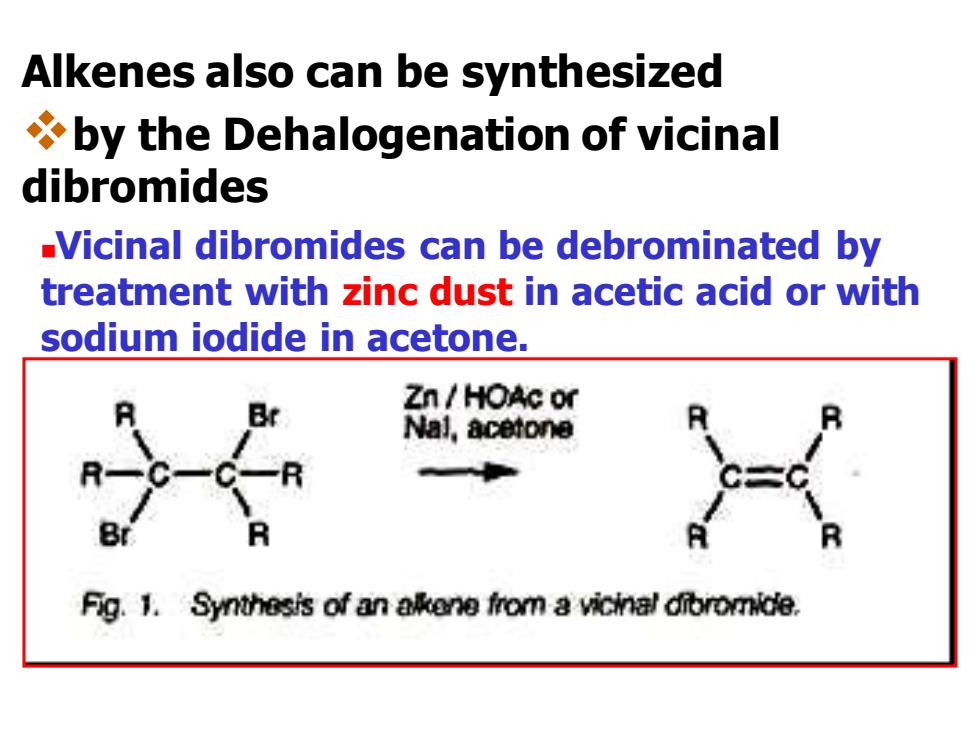
Alkenes also can be synthesized by the Dehalogenation of vicinal dibromides Vicinal dibromides can be debrominated by treatment with zinc dust in acetic acid or with sodium iodide in acetone. Zn HOAc or Nal,acetone Fig.1.Synthesis of an alkene from a vicinal dibromide
Alkenes also can be synthesized ❖by the Dehalogenation of vicinal dibromides ◼Vicinal dibromides can be debrominated by treatment with zinc dust in acetic acid or with sodium iodide in acetone
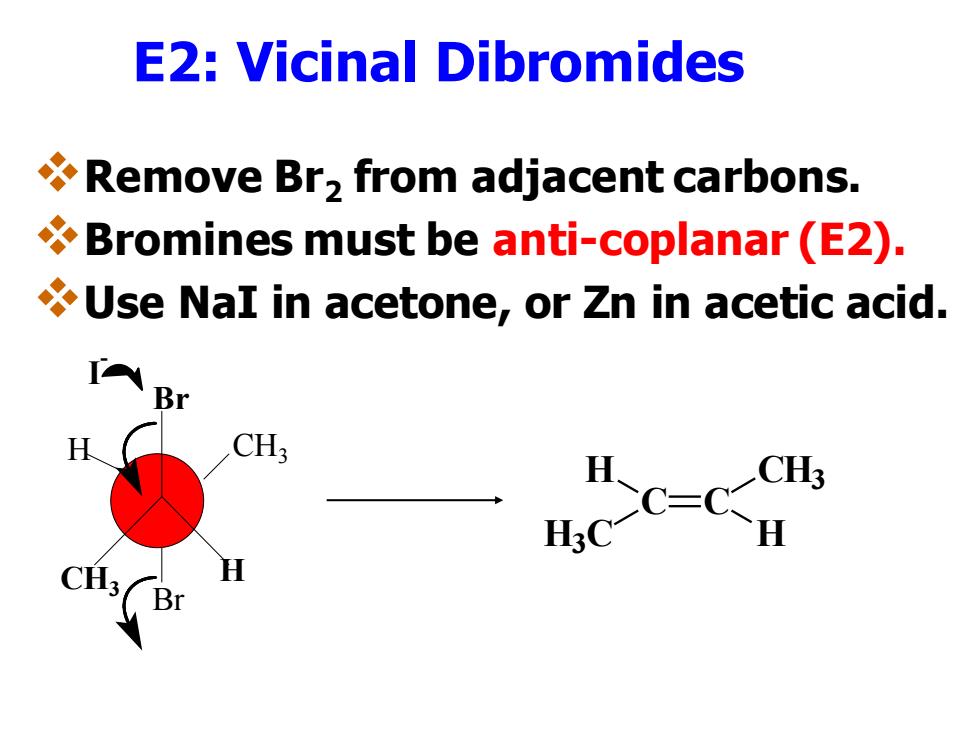
E2:Vicinal Dibromides Remove Br>from adjacent carbons. Bromines must be anti-coplanar (E2). Use NaI in acetone,or Zn in acetic acid. Br CH3 H:C C=
E2: Vicinal Dibromides ❖Remove Br2 from adjacent carbons. ❖Bromines must be anti-coplanar (E2). ❖Use NaI in acetone, or Zn in acetic acid. I - Br CH3 H Br H CH3 C C CH3 H H H3 C