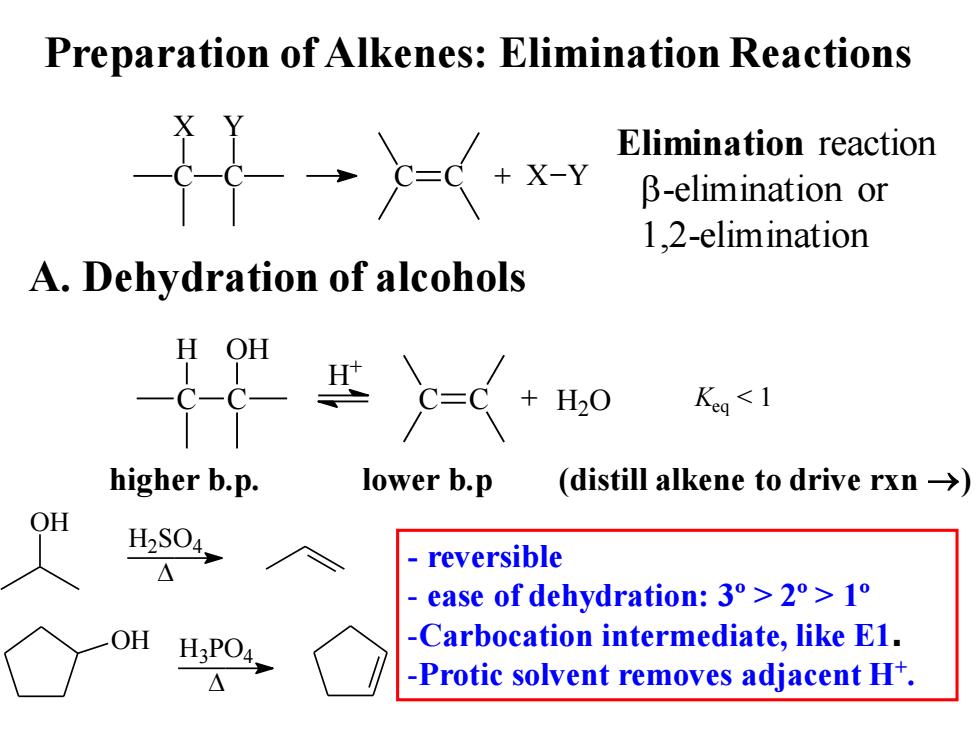
Preparation of Alkenes:Elimination Reactions 礼 Elimination reaction x-Y β-elimination or 1.2-elimination A.Dehydration of alcohols Keg<1 higher b.p. lower b.p (distill alkene to drive rxn->) OH H2S04, reversible ease of dehydration:3°>2°>1o OH H3PO4. -Carbocation intermediate,like E1. △ -Protic solvent removes adjacent H+
Preparation of Alkenes: Elimination Reactions A. Dehydration of alcohols C X C Y C C + X Y Elimination reaction b-elimination or 1,2-elimination C H C OH C C + H2O H + Keq < 1 higher b.p. lower b.p (distill alkene to drive rxn →) OH H2SO4 OH H3PO4 - reversible - ease of dehydration: 3º > 2º > 1º -Carbocation intermediate, like E1. -Protic solvent removes adjacent H+
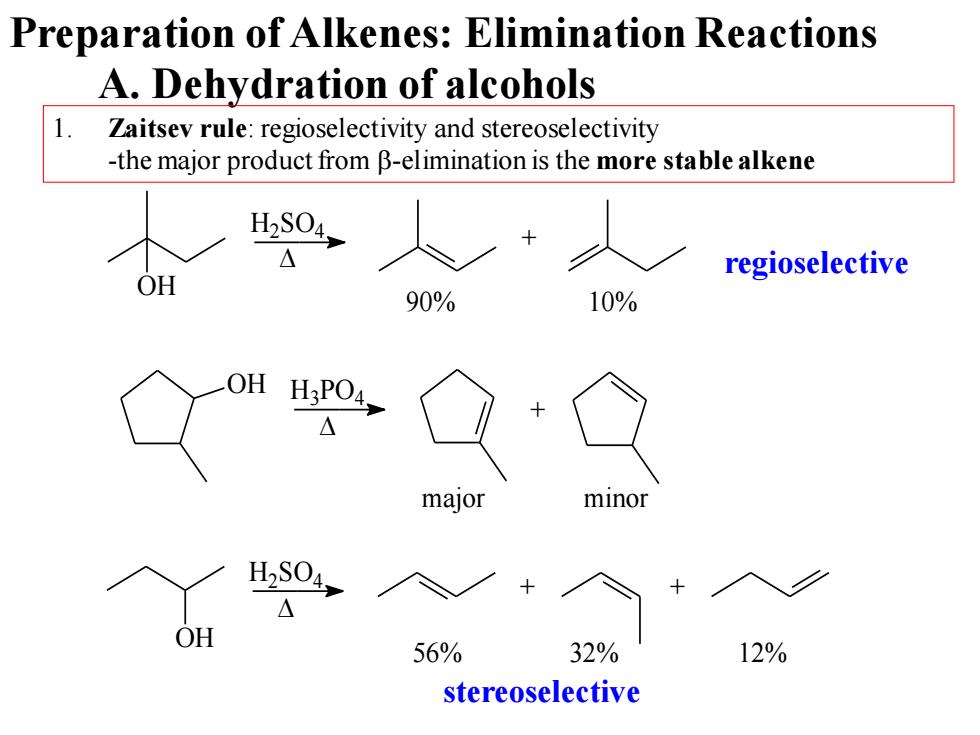
Preparation of Alkenes:Elimination Reactions A.Dehydration of alcohols 1. Zaitsev rule:regioselectivity and stereoselectivity -the major product from B-elimination is the more stable alkene 40人 regioselective OH 90% 10% H:PO4, △ major H2S04, OH 56% 32% 12% stereoselective
A. Dehydration of alcohols 1. Zaitsev rule: regioselectivity and stereoselectivity -the major product from b-elimination is the more stable alkene OH + H2SO4 90% 10% OH H3PO4 + OH + + H2SO4 56% 32% 12% major minor regioselective stereoselective Preparation of Alkenes: Elimination Reactions
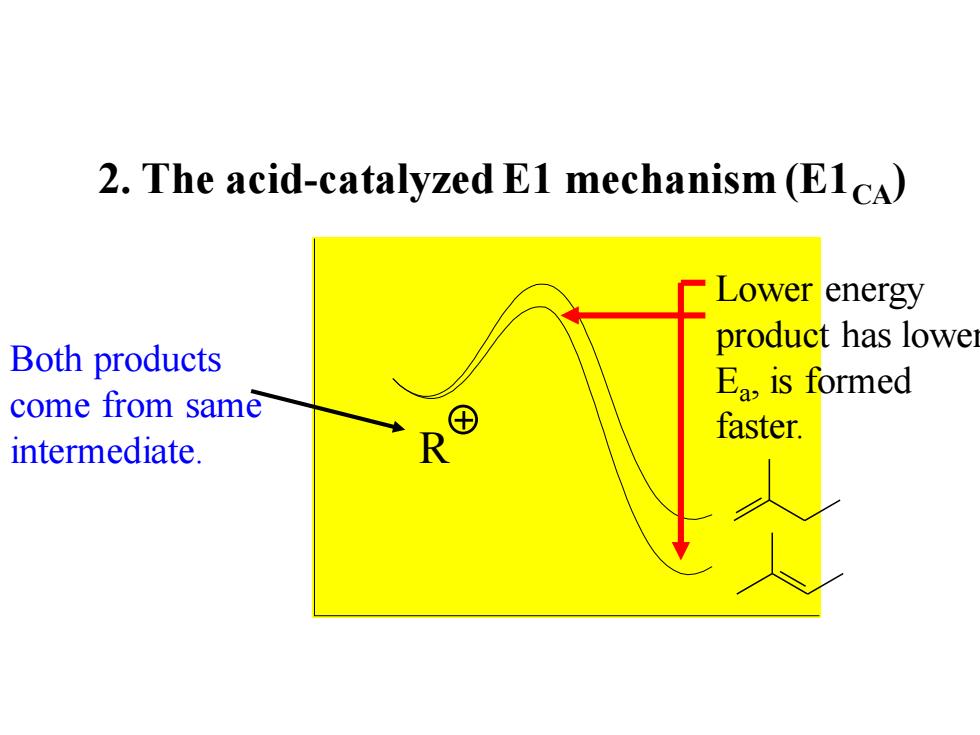
2.The acid-catalyzed E1 mechanism (E1cA) CLower e energy product has lower Both products Ea is formed come from same faster. intermediate
2. The acid-catalyzed E1 mechanism (E1CA) R Both products come from same intermediate. Lower energy product has lower Ea , is formed faster
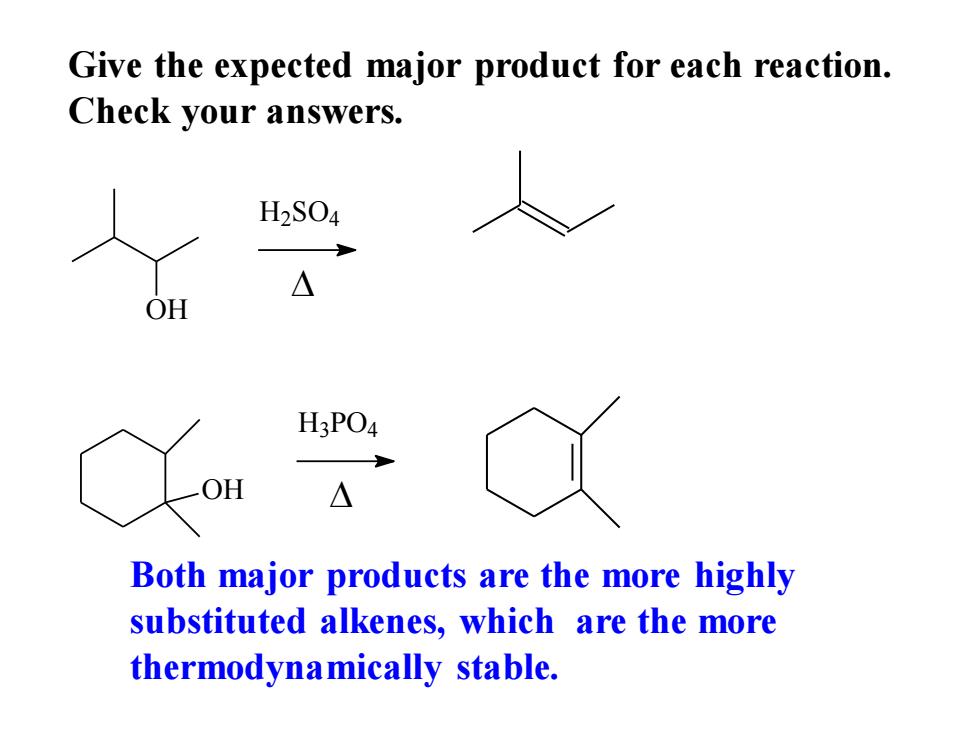
Give the expected major product for each reaction. Check your answers. H2S04 △ OH H3PO4 OH △ Both major products are the more highly substituted alkenes,which are the more thermodynamically stable
Give the expected major product for each reaction. Check your answers. OH H2 SO4 OH H3 PO4 Both major products are the more highly substituted alkenes, which are the more thermodynamically stable
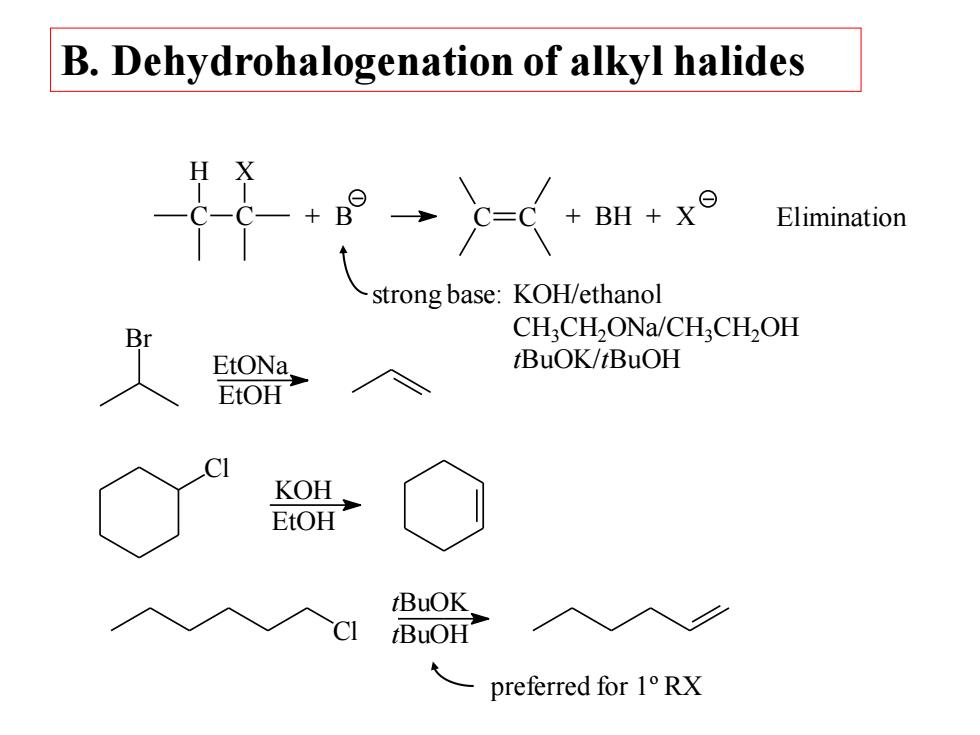
B.Dehydrohalogenation of alkyl halides BH+x⊙ Elimination strong base:KOH/ethanol CH;CH2ONa/CHCH,OH EtONa tBuOK/tBuOH EtOH KOH 州 EtOH tBuOK BuOH preferred for 1°RX
B. Dehydrohalogenation of alkyl halides C H C X + B C C + BH + X strong base: KOH/ethanol CH3CH2ONa/CH3CH2OH tBuOK/tBuOH Br EtONa EtOH Cl KOH EtOH Cl tBuOK tBuOH preferred for 1º RX Elimination