Industrial Methods Catalytic cracking of petroleum -Long-chain alkane is heated with a catalyst to produce an alkene and shorter alkane. Complex mixtures are produced. Dehydrogenation of alkanes -Hydrogen(H2)is removed with heat,catalyst. Reaction is endothermic,but entropy-favored. Neither method is suitable for lab synthesis
Industrial Methods ❖Catalytic cracking of petroleum ◼ Long-chain alkane is heated with a catalyst to produce an alkene and shorter alkane. ◼ Complex mixtures are produced. ❖Dehydrogenation of alkanes ◼ Hydrogen (H2) is removed with heat, catalyst. ◼ Reaction is endothermic, but entropy-favored. ❖Neither method is suitable for lab synthesis
Sec 3 Reaction of alkenes Reactivity of C=C Electrons in pi bond are loosely held. -Electrophiles are attracted to the pi electrons. -Carbocation intermediate forms. *Electrophilic Addition Free-Radical Addition of HBr *Reduction and oxidation
Sec 3 Reaction of alkenes ❖Reactivity of C=C ◼ Electrons in pi bond are loosely held. ◼ Electrophiles are attracted to the pi electrons. ◼ Carbocation intermediate forms. ❖Electrophilic Addition ❖Free-Radical Addition of HBr ❖Reduction and oxidation
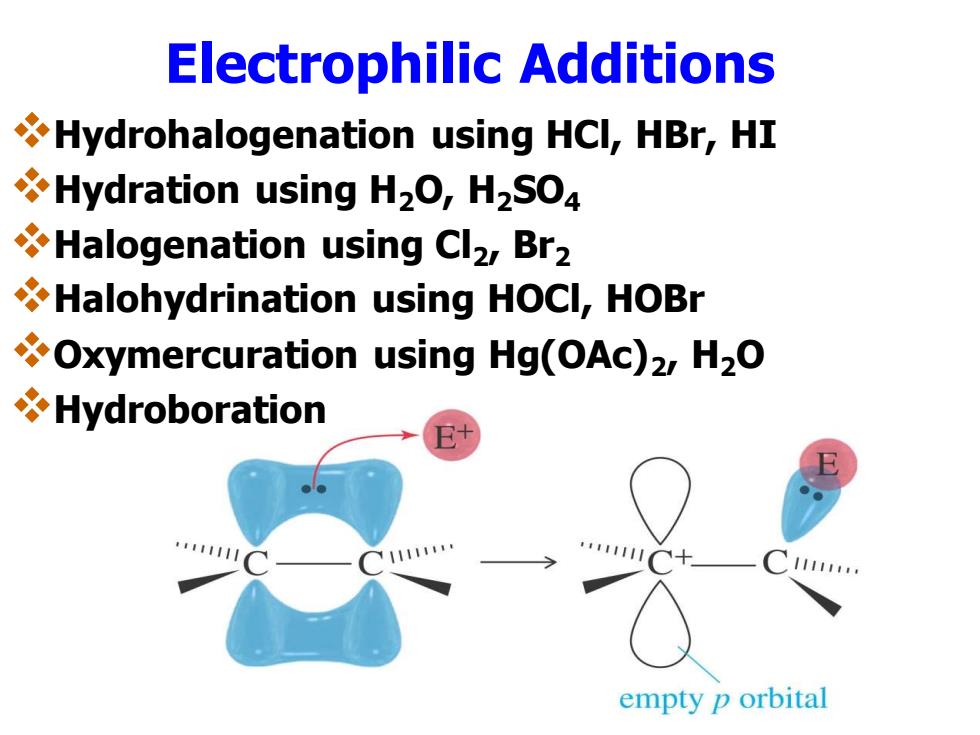
Electrophilic Additions Hydrohalogenation using HCl,HBr,HI Hydration using H2O,H2SO4 Halogenation using Cl2,Br2 Halohydrination using HOCl,HOBr Oxymercuration using Hg(OAc)2,H2O Hydroboration 11111 empty p orbital
Electrophilic Additions ❖Hydrohalogenation using HCl, HBr, HI ❖Hydration using H2O, H2SO4 ❖Halogenation using Cl2 , Br2 ❖Halohydrination using HOCl, HOBr ❖Oxymercuration using Hg(OAc)2 , H2O ❖Hydroboration
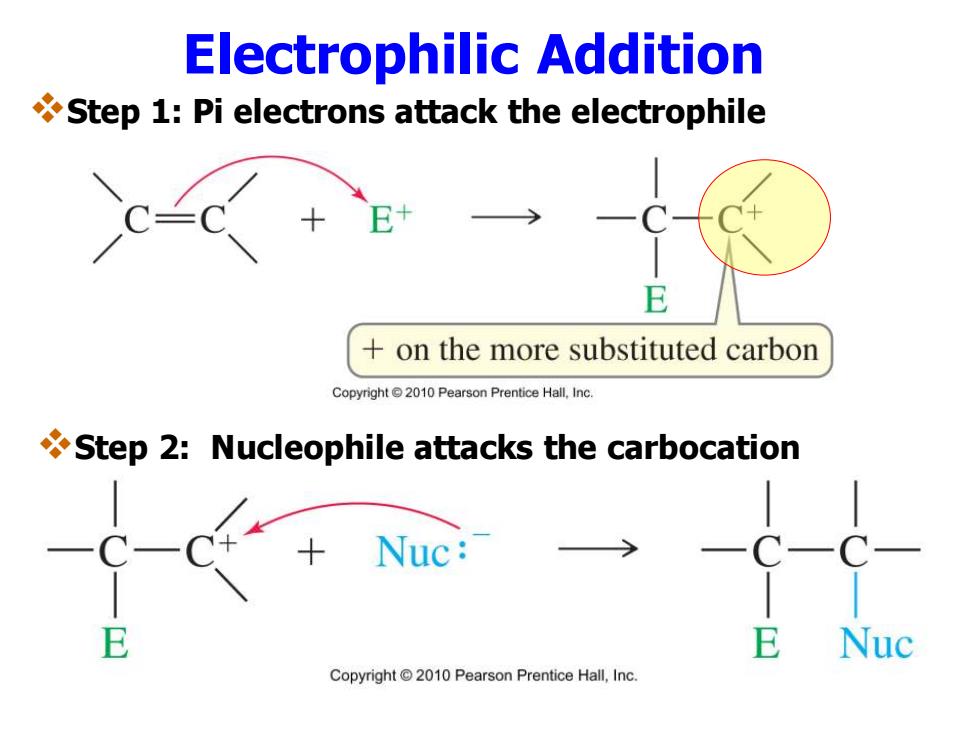
Electrophilic Addition Step 1:Pi electrons attack the electrophile CE+ C— +on the more substituted carbon Copyright 2010 Pearson Prentice Hall,Inc Step 2:Nucleophile attacks the carbocation Nuc: Copyright2010 Pearson Prentice Hall,Inc
Electrophilic Addition ❖Step 1: Pi electrons attack the electrophile ❖Step 2: Nucleophile attacks the carbocation
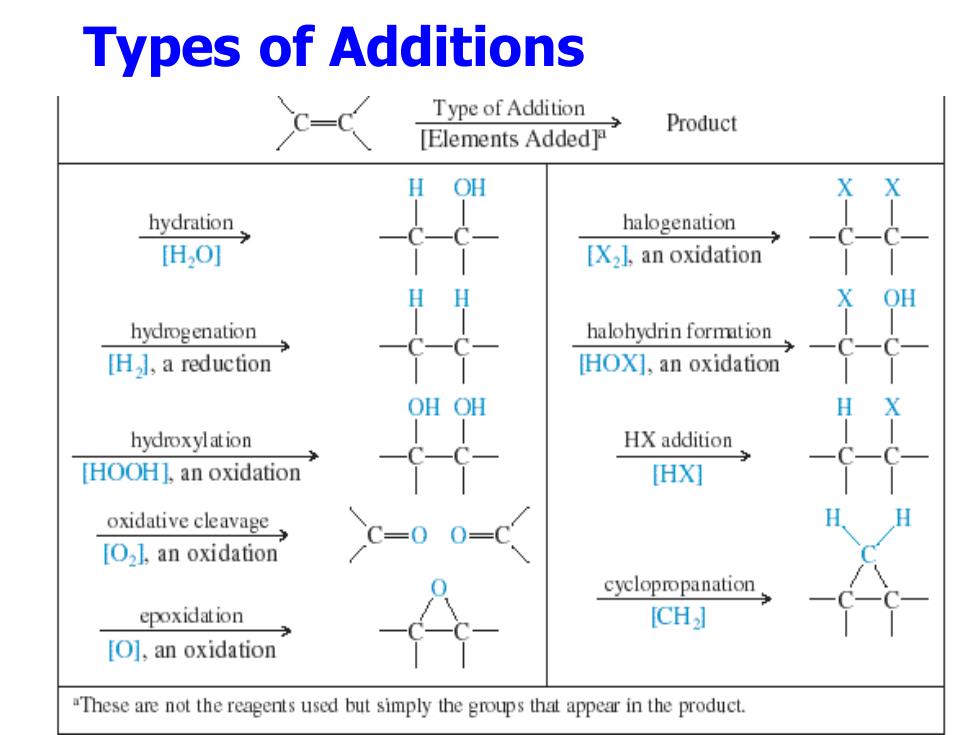
Types of Additions C- Type of Addition Product [Elements Added] OH hydration halogenation H,0] [X2],an oxidation HH X OH hydrogenation halohydrin formation H】,a reduction HOX],an oxidation OHOH hydroxylation HX addition [HOOH].an oxidation [HX] oxidative cleavage C=0 [O2l,an oxidation cyclopropanation epoxidation [CH] [O],an oxidation "These are not the reagents used but simply the groups that appear in the product
Types of Additions