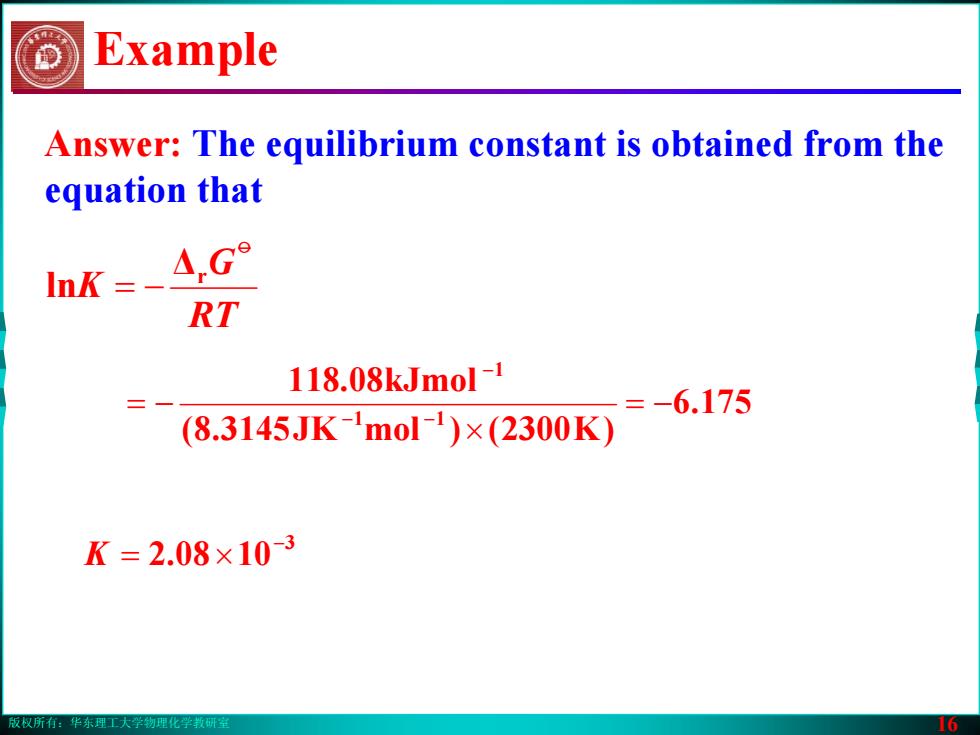
版权所有:华东理工大学物理化学教研室 16 Answer: The equilibrium constant is obtained from the equation that 175.6 )K2300()molJK3145.8( 118.08kJmol 11 1 −= × −= −− − 3 1008.2 − K ×= Example RT G K o Δr ln −=
版权所有:华东理工大学物理化学教研室 16 Answer: The equilibrium constant is obtained from the equation that 175.6 )K2300()molJK3145.8( 118.08kJmol 11 1 −= × −= −− − 3 1008.2 − K ×= Example RT G K o Δr ln −=
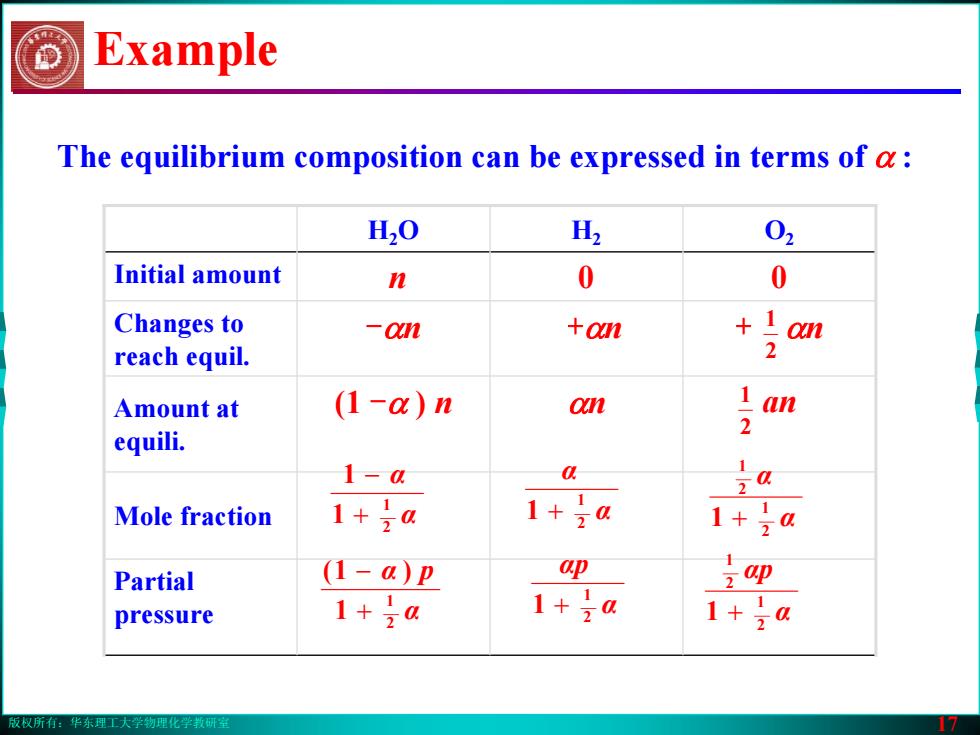
版权所有:华东理工大学物理化学教研室 17 The equilibrium composition can be expressed in terms of α : H2O H2 O2 Initial amount n 0 0 Changes to reach equil. -αn +αn + αn Amount at equili. (1 -α ) n αn an Mole fraction Partial pressure Example α α 2 1 1 1 + − α α 2 1 1 + α α 2 1 2 1 1 + α α p 2 1 1 )1( + − α αp 2 1 1 + α αp 2 1 2 1 1 + 2 1 2 1
版权所有:华东理工大学物理化学教研室 17 The equilibrium composition can be expressed in terms of α : H2O H2 O2 Initial amount n 0 0 Changes to reach equil. -αn +αn + αn Amount at equili. (1 -α ) n αn an Mole fraction Partial pressure Example α α 2 1 1 1 + − α α 2 1 1 + α α 2 1 2 1 1 + α α p 2 1 1 )1( + − α αp 2 1 1 + α αp 2 1 2 1 1 + 2 1 2 1