
版权所有:华东理工大学物理化学教研室 1 Physical Chemistry Peter Atkins Peter Atkins (Sixth edition) (Sixth edition) Bilingual Program
版权所有:华东理工大学物理化学教研室 1 Physical Chemistry Peter Atkins Peter Atkins (Sixth edition) (Sixth edition) Bilingual Program

版权所有:华东理工大学物理化学教研室 2 Part 1: Equilibrium Bilingual Program 3. The First Law: the machinery
版权所有:华东理工大学物理化学教研室 2 Part 1: Equilibrium Bilingual Program 3. The First Law: the machinery
版权所有:华东理工大学物理化学教研室 3 In this chapter we begin to unfold some of the power of thermodynamics by showing how to establish relations between different properties of a system. The procedure we use is based on the experimental fact that the internal energy and the enthalpy are state functions, and we derive a number of relations between observables by exploring the mathematical consequences of these facts. 3. The First Law: the machinery
版权所有:华东理工大学物理化学教研室 3 In this chapter we begin to unfold some of the power of thermodynamics by showing how to establish relations between different properties of a system. The procedure we use is based on the experimental fact that the internal energy and the enthalpy are state functions, and we derive a number of relations between observables by exploring the mathematical consequences of these facts. 3. The First Law: the machinery
版权所有:华东理工大学物理化学教研室 4 3.1 State functions 1). Exact and inexact differentials 2). Changes in internal energy 3). Expansion coefficient 3.2 The temperature dependence of the enthalpy 1). Changes in enthalpy at constant volume 2). The isothermal compressibility 3). The Joule -Thomson effect 3.3 The reaction between Cv and Cp 3. The First Law: the machinery
版权所有:华东理工大学物理化学教研室 4 3.1 State functions 1). Exact and inexact differentials 2). Changes in internal energy 3). Expansion coefficient 3.2 The temperature dependence of the enthalpy 1). Changes in enthalpy at constant volume 2). The isothermal compressibility 3). The Joule -Thomson effect 3.3 The reaction between Cv and Cp 3. The First Law: the machinery
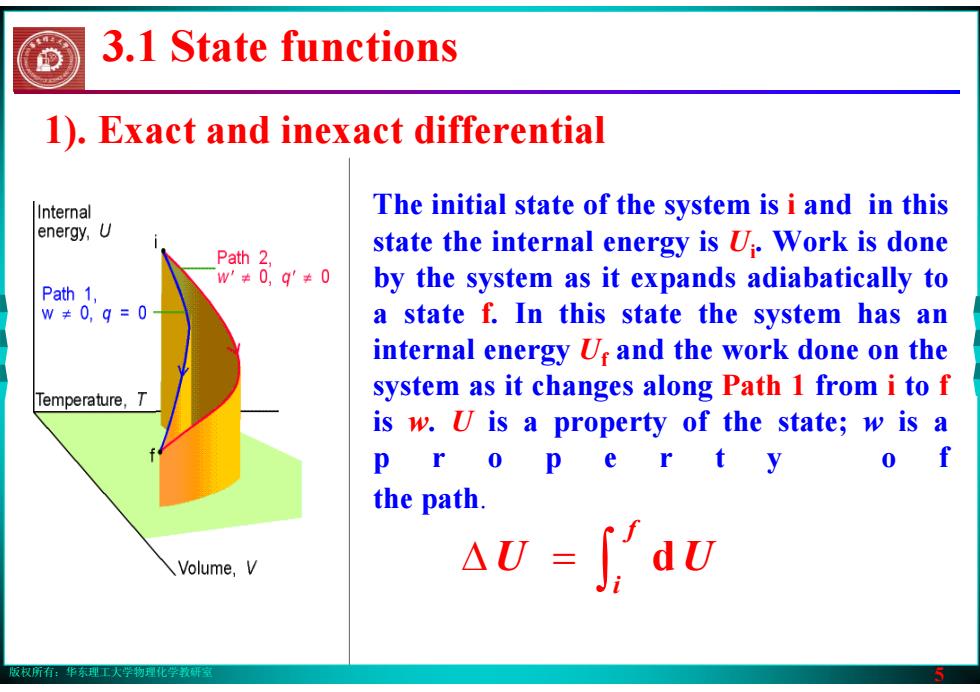
版权所有:华东理工大学物理化学教研室 5 The initial state of the system is i and in this state the internal energy is Ui. Work is done by the system as it expands adiabatically to a state f. In this state the system has an internal energy Uf and the work done on the system as it changes along Path 1 from i to f is w. U is a property of the state; w is a p r o p e r t y o f the path. 3.1 State functions 1). Exact and inexact differential ∫ Δ = f i U d U
版权所有:华东理工大学物理化学教研室 5 The initial state of the system is i and in this state the internal energy is Ui. Work is done by the system as it expands adiabatically to a state f. In this state the system has an internal energy Uf and the work done on the system as it changes along Path 1 from i to f is w. U is a property of the state; w is a p r o p e r t y o f the path. 3.1 State functions 1). Exact and inexact differential ∫ Δ = f i U d U