
版权所有:华东理工大学物理化学教研室 1 Physical Chemistry Peter Atkins Peter Atkins (Sixth edition) (Sixth edition) Bilingual Program
版权所有:华东理工大学物理化学教研室 1 Physical Chemistry Peter Atkins Peter Atkins (Sixth edition) (Sixth edition) Bilingual Program

版权所有:华东理工大学物理化学教研室 2 Part 1: Equilibrium Bilingual Program 5. The Second Law:the machinery
版权所有:华东理工大学物理化学教研室 2 Part 1: Equilibrium Bilingual Program 5. The Second Law:the machinery
版权所有:华东理工大学物理化学教研室 3 In this chapter: First: to find relations between properties that might not be thought to be related; to derive expressions for the variation of the G with T and p. Second: to introduce the chemical potential, a property that will be at the center of discussions in the remaining chapters of this part of the text; to derive expression of fugacity. The 'chemical potential', the quantity on which almost all the most important applications of thermodynamics to chemistry are based. 5. The Second Law: the machinery
版权所有:华东理工大学物理化学教研室 3 In this chapter: First: to find relations between properties that might not be thought to be related; to derive expressions for the variation of the G with T and p. Second: to introduce the chemical potential, a property that will be at the center of discussions in the remaining chapters of this part of the text; to derive expression of fugacity. The 'chemical potential', the quantity on which almost all the most important applications of thermodynamics to chemistry are based. 5. The Second Law: the machinery
版权所有:华东理工大学物理化学教研室 4 Combing the First and Second Laws 5.1 Properties of the internal energy 5.2 Properties of the Gibbs energy 5.3 The chemical potential of a pure substance Real gases:the fugacity 5.4 The definition of fugacity 5.5 Standard states of real gases 5.6 The relation between fugacity and pressure 5. The Second Law: the machinery
版权所有:华东理工大学物理化学教研室 4 Combing the First and Second Laws 5.1 Properties of the internal energy 5.2 Properties of the Gibbs energy 5.3 The chemical potential of a pure substance Real gases:the fugacity 5.4 The definition of fugacity 5.5 Standard states of real gases 5.6 The relation between fugacity and pressure 5. The Second Law: the machinery
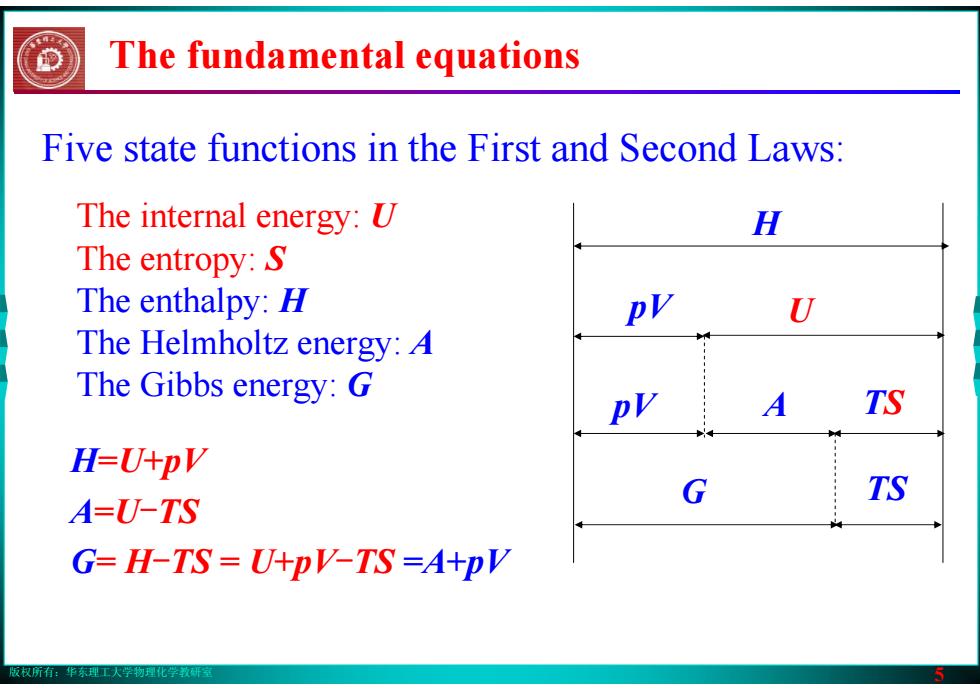
版权所有:华东理工大学物理化学教研室 5 The fundamental equations Five state functions in the First and Second Laws: The internal energy: U The entropy: S The enthalpy: H The Helmholtz energy: A The Gibbs energy: G H pV U pV A TS G TS H=U+pV A=U-TS G= H-TS = U+pV-TS =A+pV
版权所有:华东理工大学物理化学教研室 5 The fundamental equations Five state functions in the First and Second Laws: The internal energy: U The entropy: S The enthalpy: H The Helmholtz energy: A The Gibbs energy: G H pV U pV A TS G TS H=U+pV A=U-TS G= H-TS = U+pV-TS =A+pV