上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Phases Diagram graph showing the limiting conditions for solid. liquid,and gaseous phases of a single substance or of a mixture of substances while undergoing changes in pressure and temperature or in some other combination of variables,such as solubility and temperature. G JIAO TONG UNIV
Phases Diagram
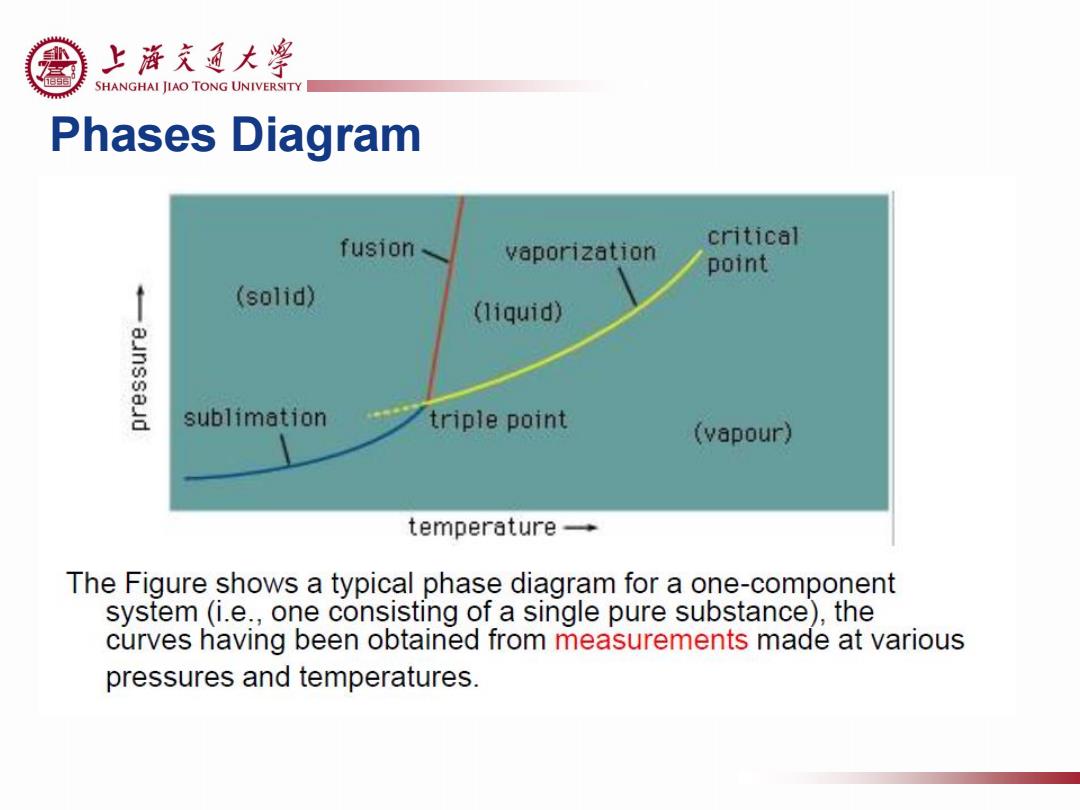
上游充通大¥ SHANGHAI JIAO TONG UNIVERSITY Phases Diagram fusion、 critical vaporization point (solid) (liquid) sublimation triple point (vapour) temperature- The Figure shows a typical phase diagram for a one-component system(i.e.,one consisting of a single pure substance),the curves having been obtained from measurements made at various pressures and temperatures
Phases Diagram

上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Phases Rule Thermodynamic stability of coexisting elements,compounds,and solution Equilibrium structure How many phases 所有多相平衡系统都遵循的普遍规律! 描述平衡系统中相数、组分数以及影响系统状态的独立 可变因素(如温度、压力、组成等)的总数(称为自由 度数)之间的关系
Phases Rule