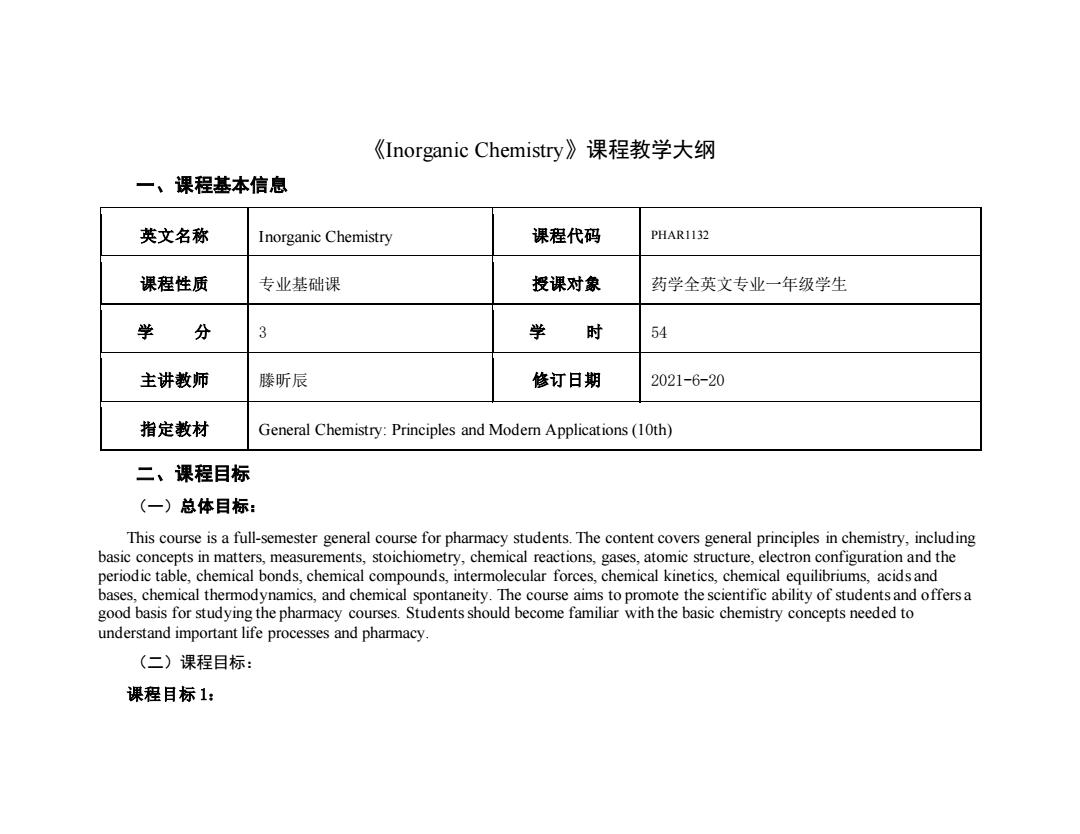
《Inorganic Chemistry》课程教学大纲 一、课程基本信息 英文名称 Inorganic Chemistry 课程代码 PHAR1132 课程性质 专业基础课 授课对象 药学全英文专业一年级学生 学 分 3 学 时 54 主讲教师 滕昕辰 修订日期 2021-6-20 指定教材 General Chemistry:Principles and Modern Applications(10th) 二、课程目标 (一)总体目标: This course is a full-semester general course for pharmacy students.The content covers general principles in chemistry,including basic concepts in matters,measurements,stoichiometry,chemical reactions,gases,atomic structure,electron configuration and the periodic table,chemical bonds,chemical compounds,intermolecular forces,chemical kinetics,chemical equilibriums,acids and bases,chemical thermodynamics,and chemical spontaneity.The course aims to promote the scientific ability of students and offersa good basis for studying the pharmacy courses.Students should become familiar with the basic chemistry concepts needed to understand important life processes and pharmacy. (二)课程目标: 课程目标1:
《Inorganic Chemistry》课程教学大纲 一、课程基本信息 英文名称 Inorganic Chemistry 课程代码 PHAR1132 课程性质 专业基础课 授课对象 药学全英文专业一年级学生 学 分 3 学 时 54 主讲教师 滕昕辰 修订日期 2021-6-20 指定教材 General Chemistry: Principles and Modern Applications (10th) 二、课程目标 (一)总体目标: This course is a full-semester general course for pharmacy students. The content covers general principles in chemistry, including basic concepts in matters, measurements, stoichiometry, chemical reactions, gases, atomic structure, electron configuration and the periodic table, chemical bonds, chemical compounds, intermolecular forces, chemical kinetics, chemical equilibriums, acids and bases, chemical thermodynamics, and chemical spontaneity. The course aims to promote the scientific ability of students and offers a good basis for studying the pharmacy courses. Students should become familiar with the basic chemistry concepts needed to understand important life processes and pharmacy. (二)课程目标: 课程目标 1:
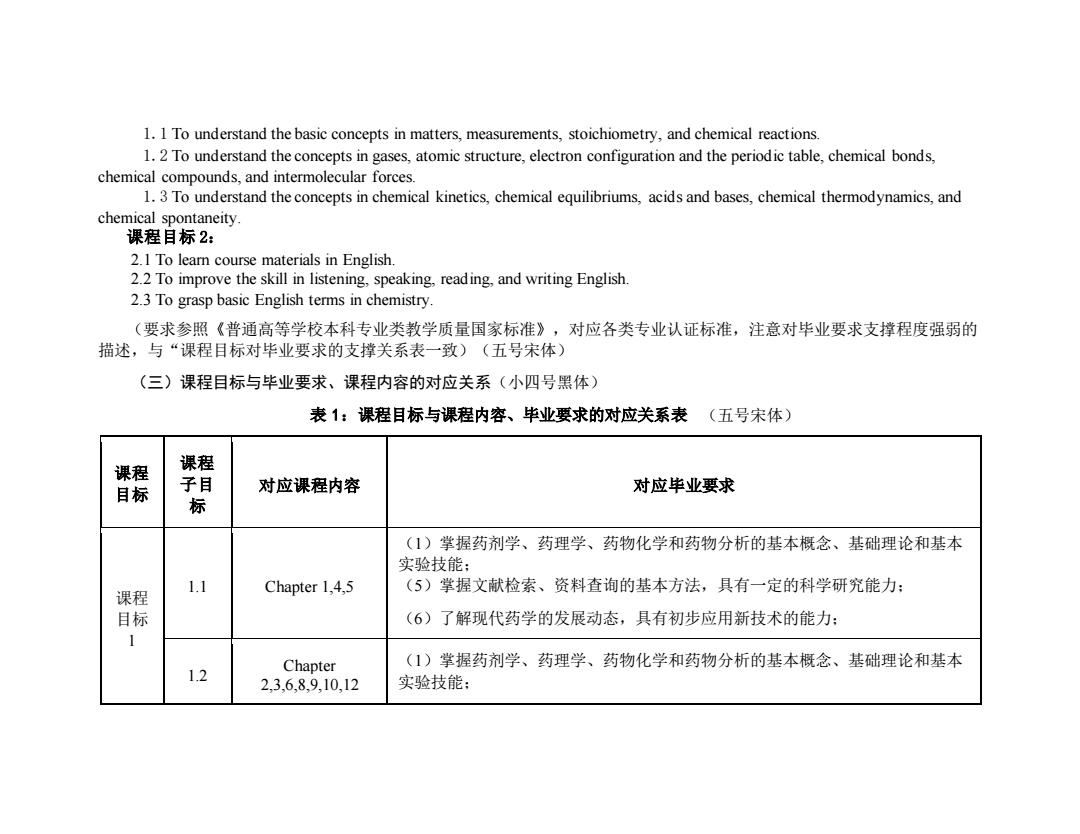
1.1 To understand the basic concepts in matters,measurements,stoichiometry,and chemical reactions. 1.2 To understand the concepts in gases,atomic structure,electron configuration and the periodic table,chemical bonds, chemical compounds,and intermolecular forces. 1.3 To understand the concepts in chemical kinetics,chemical equilibriums,acids and bases,chemical thermodynamics,and chemical spontaneity. 课程目标2: 2.1 To learn course materials in English. 2.2 To improve the skill in listening,speaking,reading,and writing English. 2.3 To grasp basic English terms in chemistry. (要求参照《普通高等学校本科专业类教学质量国家标准》,对应各类专业认证标准,注意对毕业要求支撑程度强弱的 描述,与“课程目标对毕业要求的支撑关系表一致)(五号宋体) (三)课程目标与毕业要求、课程内容的对应关系(小四号黑体) 表1:课程目标与课程内容、毕业要求的对应关系表(五号宋体) 课程 课程 目标 子目 对应课程内容 对应毕业要求 标 (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 实验技能: 1.1 Chapter 1,4,5 (5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力: 课程 目标 (6)了解现代药学的发展动态,具有初步应用新技术的能力: 1 Chapter (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 1.2 23.6,89,10,12 实验技能:
1.1 To understand the basic concepts in matters, measurements, stoichiometry, and chemical reactions. 1.2 To understand the concepts in gases, atomic structure, electron configuration and the periodic table, chemical bonds, chemical compounds, and intermolecular forces. 1.3 To understand the concepts in chemical kinetics, chemical equilibriums, acids and bases, chemical thermodynamics, and chemical spontaneity. 课程目标 2: 2.1 To learn course materials in English. 2.2 To improve the skill in listening, speaking, reading, and writing English. 2.3 To grasp basic English terms in chemistry. (要求参照《普通高等学校本科专业类教学质量国家标准》,对应各类专业认证标准,注意对毕业要求支撑程度强弱的 描述,与“课程目标对毕业要求的支撑关系表一致)(五号宋体) (三)课程目标与毕业要求、课程内容的对应关系(小四号黑体) 表 1:课程目标与课程内容、毕业要求的对应关系表 (五号宋体) 课程 目标 课程 子目 标 对应课程内容 对应毕业要求 课程 目标 1 1.1 Chapter 1,4,5 (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 实验技能; (5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力; (6)了解现代药学的发展动态,具有初步应用新技术的能力; 1.2 Chapter 2,3,6,8,9,10,12 (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 实验技能;
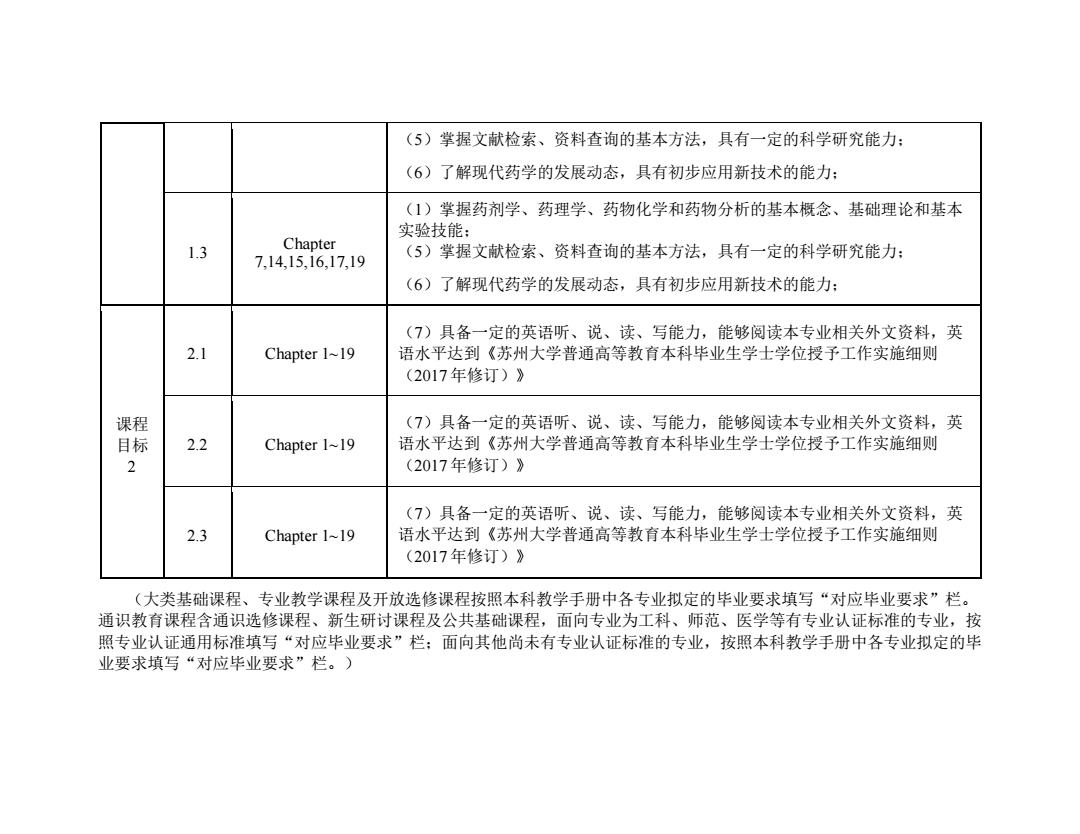
(5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力: (6)了解现代药学的发展动态,具有初步应用新技术的能力: (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 实验技能: 1.3 Chapter (5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力: 7,14,15,16,17,19 (6)了解现代药学的发展动态,具有初步应用新技术的能力: (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 2.1 Chapter 1~19 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 (2017年修订)》 课程 (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 目标 2.2 Chapter 1~19 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 2 (2017年修订)》 (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 2.3 Chapter 1~19 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 (2017年修订)》 (大类基础课程、专业教学课程及开放选修课程按照本科教学手册中各专业拟定的毕业要求填写“对应毕业要求”栏。 通识教育课程含通识选修课程、新生研讨课程及公共基础课程,面向专业为工科、师范、医学等有专业认证标准的专业,按 照专业认证通用标准填写“对应毕业要求”栏:面向其他尚未有专业认证标准的专业,按照本科教学手册中各专业拟定的毕 业要求填写“对应毕业要求”栏。)
(5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力; (6)了解现代药学的发展动态,具有初步应用新技术的能力; 1.3 Chapter 7,14,15,16,17,19 (1)掌握药剂学、药理学、药物化学和药物分析的基本概念、基础理论和基本 实验技能; (5)掌握文献检索、资料查询的基本方法,具有一定的科学研究能力; (6)了解现代药学的发展动态,具有初步应用新技术的能力; 课程 目标 2 2.1 Chapter 1~19 (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 (2017 年修订)》 2.2 Chapter 1~19 (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 (2017 年修订)》 2.3 Chapter 1~19 (7)具备一定的英语听、说、读、写能力,能够阅读本专业相关外文资料,英 语水平达到《苏州大学普通高等教育本科毕业生学士学位授予工作实施细则 (2017 年修订)》 (大类基础课程、专业教学课程及开放选修课程按照本科教学手册中各专业拟定的毕业要求填写“对应毕业要求”栏。 通识教育课程含通识选修课程、新生研讨课程及公共基础课程,面向专业为工科、师范、医学等有专业认证标准的专业,按 照专业认证通用标准填写“对应毕业要求”栏;面向其他尚未有专业认证标准的专业,按照本科教学手册中各专业拟定的毕 业要求填写“对应毕业要求”栏。)

三、教学内容 Chapter 1.Matter:Its Properties and Measurement 1.教学目标 Learn how to use the scientific methods to study a subject Be able to classify matters ● Understand the general properties of different matters Know how to make measurements properly 2. 教学重难点 教学重点: Definition,properties and classification of matters Measurement ofmatters 教学难点 Classification of matters Unit conversion Uncertainties in scientific measurements Significant figures in calculations 3. 教学内容 1. The Scientific Method How to study chemistry The definition of hypothesis,law and theory 2.Properties of Matter Physical properties and physical changes Chemical properties and chemical changes Extensive and intensive properties 3.Classification of Matter Identification of matter Matter at the atomic level 4.Measurement of Matter:SI(Metric)Units
三、教学内容 Chapter 1. Matter: Its Properties and Measurement 1. 教学目标 • Learn how to use the scientific methods to study a subject • Be able to classify matters • Understand the general properties of different matters • Know how to make measurements properly 2. 教学重难点 教学重点: • Definition, properties and classification of matters • Measurement of matters 教学难点: • Classification of matters • Unit conversion • Uncertainties in scientific measurements • Significant figures in calculations 3. 教学内容 1. The Scientific Method How to study chemistry The definition of hypothesis, law and theory 2. Properties of Matter Physical properties and physical changes Chemical properties and chemical changes Extensive and intensive properties 3. Classification of Matter Identification of matter Matter at the atomic level 4. Measurement of Matter: SI (Metric) Units

Measurements are quantitative observations SI base units and their definition Derived SI units Rules of using SI units Converting units 5.Density and Percent Composition:Their Use in Problem Solving Measuring and calculating density Calculating percent composition 6.Uncertainties in Scientific Measurements Definition of uncertainty Precision and accuracy 1-7 Significant Figures Definition of significant figures Significant figures in calculations 4. 教学方法 多媒体教学: Illustration ofscientific methods Illustration ofclassification of matters Illustration ofmatterat microscopic level ● Illustration ofdifferent types of measurements Illustration ofsignificant figure in calculations 互动教学: What kind of matter is...(air,oxygen,tap water,distilled water,soda,woods)? Why need to standardize units? How to achieve precision and accuracy? How to determine uncertainty in measurement? How to maintain the precision of measurement during calculation? 5. 教学评价 课后问题:
Measurements are quantitative observations SI base units and their definition Derived SI units Rules of using SI units Converting units 5. Density and Percent Composition: Their Use in Problem Solving Measuring and calculating density Calculating percent composition 6. Uncertainties in Scientific Measurements Definition of uncertainty Precision and accuracy 1-7 Significant Figures Definition of significant figures Significant figures in calculations 4. 教学方法 多媒体教学: • Illustration of scientific methods • Illustration of classification of matters • Illustration of matter at microscopic level • Illustration of different types of measurements • Illustration of significant figure in calculations 互动教学: • What kind of matter is … (air, oxygen, tap water, distilled water, soda, woods)? • Why need to standardize units? • How to achieve precision and accuracy? • How to determine uncertainty in measurement? • How to maintain the precision of measurement during calculation? 5. 教学评价 课后问题: