
Bredt's Rule 中 A bridged bicyclic compound cannot have a double bond at a bridgehead position unless one of the rings contains at least eight carbon atoms. Examples: Stable.Double bond in 8-membered ring. Unstable. Violates Bredt's rule
Bredt’s Rule ❖A bridged bicyclic compound cannot have a double bond at a bridgehead position unless one of the rings contains at least eight carbon atoms. ❖Examples: Unstable. Violates Bredt’s rule Stable. Double bond in 8-membered ring
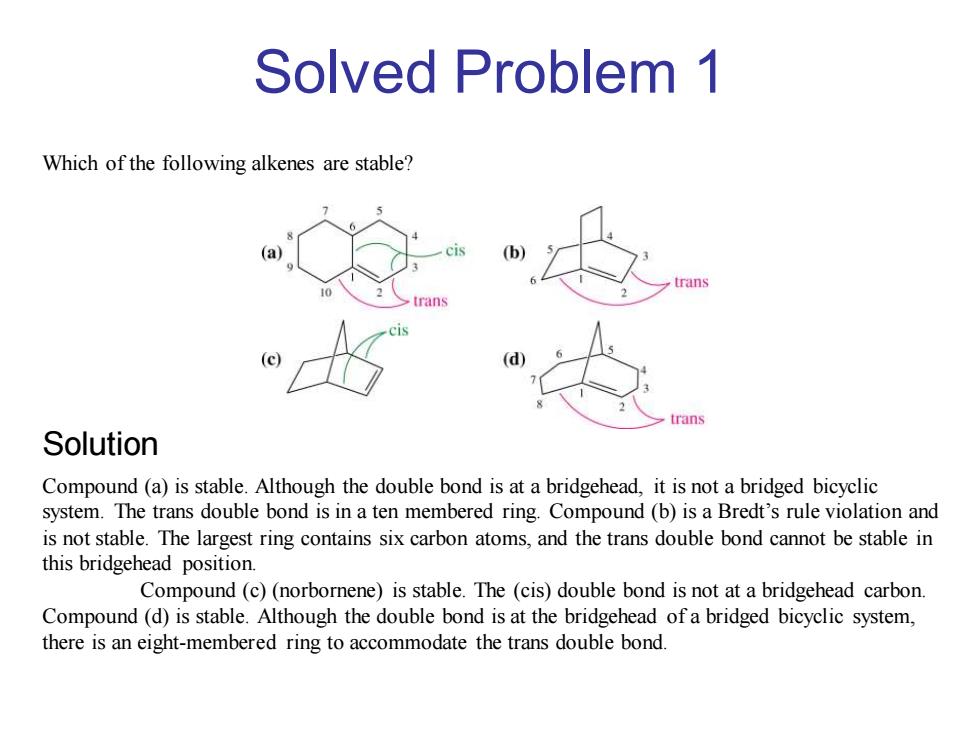
Solved Problem 1 Which of the following alkenes are stable? rans trans Solution Compound(a)is stable.Although the double bond is at a bridgehead,it is not a bridged bicyclic system.The trans double bond is in a ten membered ring.Compound(b)is a Bredt's rule violation and is not stable.The largest ring contains six carbon atoms,and the trans double bond cannot be stable in this bridgehead position. Compound(c)(norbornene)is stable.The (cis)double bond is not at a bridgehead carbon. Compound(d)is stable.Although the double bond is at the bridgehead of a bridged bicyclic system, there is an eight-membered ring to accommodate the trans double bond
Compound (a) is stable. Although the double bond is at a bridgehead, it is not a bridged bicyclic system. The trans double bond is in a ten membered ring. Compound (b) is a Bredt’s rule violation and is not stable. The largest ring contains six carbon atoms, and the trans double bond cannot be stable in this bridgehead position. Compound (c) (norbornene) is stable. The (cis) double bond is not at a bridgehead carbon. Compound (d) is stable. Although the double bond is at the bridgehead of a bridged bicyclic system, there is an eight-membered ring to accommodate the trans double bond. Which of the following alkenes are stable? Solved Problem 1 Solution

Sec 2 Preparation of alkenes Alkenes can be synthesized by the reduction of alkynes,or by the elimination of alkyl halides and alcohols. -E2 dehydrohalogenation (-HX);E1 dehydrohalogenation (-HX);Dehydration of alcohols (-H2O) Dehalogenation of vicinal dibromides(-X2)
Sec 2 Preparation of alkenes Alkenes can be synthesized ❖by the reduction of alkynes, or ❖by the elimination of alkyl halides and alcohols. ◼E2 dehydrohalogenation (-HX);E1 dehydrohalogenation (-HX);Dehydration of alcohols (-H2O) ❖Dehalogenation of vicinal dibromides(-X2 )

Reduction of alkynes Lindlar's催化剂法: H5 C2HsC=CC2H5 Pd/BaSO. H 喹啉 顺式烯烃 硼氢化方法 C2H5 C2H5-C=C-CH3+1/2BH3)2 C2H5 CH3 顺式烯烃 H Z-2-戊烯 液氨法 CH3-C=C-CH3 2Na+NH 液氨 2NaNH2 CH3 反式烯烃
顺式烯烃 C2H5 C CC2H5 + H2 Pd/BaSO4 喹啉 C C H5 C2 C2 H5 H H Lindlar’s催化剂法: 硼氢化方法 C2 H5 C C CH3 + 1/2(BH3 )2 o 0 C C C C2H5 CH3 H B o 0 C CH3COOH C C C2H5 CH3 H H Z-2-戊烯 顺式烯烃 Reduction of alkynes CH3 C C CH3 + 2Na + NH3 液氨 C C + 2NaNH2 H H CH3 H3C 反式烯烃 液氨法
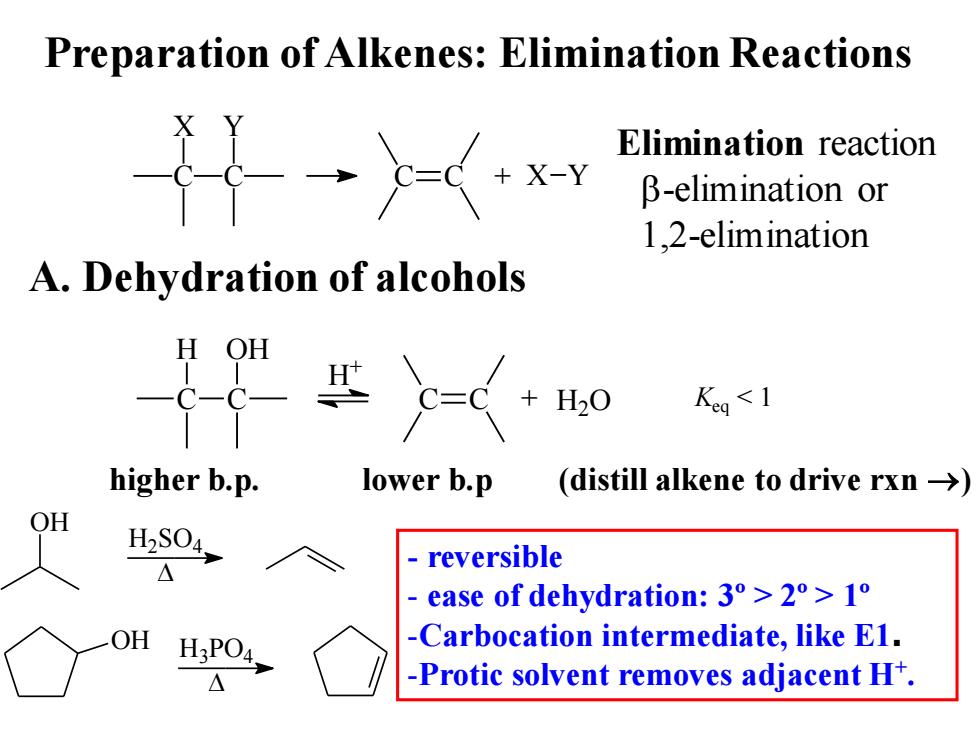
Preparation of Alkenes:Elimination Reactions 礼 Elimination reaction x-Y β-elimination or 1.2-elimination A.Dehydration of alcohols Keg<1 higher b.p. lower b.p (distill alkene to drive rxn->) OH H2S04, reversible ease of dehydration:3°>2°>1o OH H3PO4. -Carbocation intermediate,like E1. △ -Protic solvent removes adjacent H+
Preparation of Alkenes: Elimination Reactions A. Dehydration of alcohols C X C Y C C + X Y Elimination reaction b-elimination or 1,2-elimination C H C OH C C + H2O H + Keq < 1 higher b.p. lower b.p (distill alkene to drive rxn →) OH H2SO4 OH H3PO4 - reversible - ease of dehydration: 3º > 2º > 1º -Carbocation intermediate, like E1. -Protic solvent removes adjacent H+