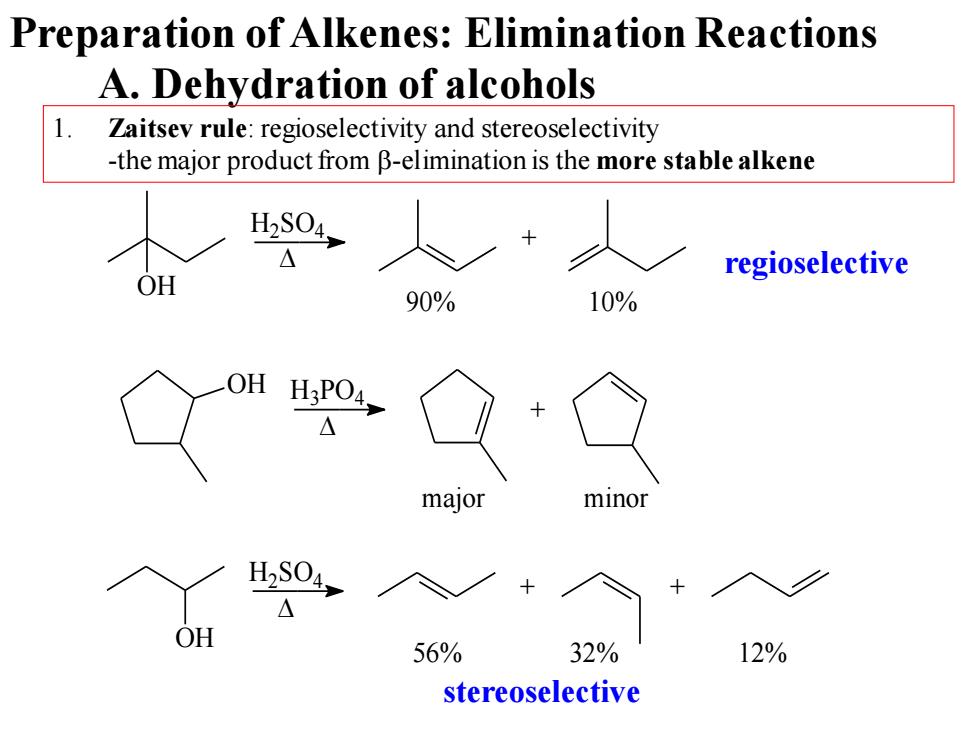
Preparation of Alkenes:Elimination Reactions A.Dehydration of alcohols 1. Zaitsev rule:regioselectivity and stereoselectivity -the major product from B-elimination is the more stable alkene 40人 regioselective OH 90% 10% H3P04, △ major H2S04, OH 56% 32% 12% stereoselective
A. Dehydration of alcohols 1. Zaitsev rule: regioselectivity and stereoselectivity -the major product from b-elimination is the more stable alkene OH + H2SO4 90% 10% OH H3PO4 + OH + + H2SO4 56% 32% 12% major minor regioselective stereoselective Preparation of Alkenes: Elimination Reactions

2.The acid-catalyzed E1 mechanism (E1cA) CLower e energy product has lower Both products Ea is formed come from same faster. intermediate
2. The acid-catalyzed E1 mechanism (E1CA) R Both products come from same intermediate. Lower energy product has lower Ea , is formed faster

Give the expected major product for each reaction. Check your answers. H2S04 OH H3PO4 OH △ Both major products are the more highly substituted alkenes,which are the more thermodynamically stable
Give the expected major product for each reaction. Check your answers. OH H2 SO4 OH H3 PO4 Both major products are the more highly substituted alkenes, which are the more thermodynamically stable

B.Dehydrohalogenation of alkyl halides BH+x⊙ Elimination strong base:KOH/ethanol CH;CH2ONa/CHCH,OH EtONa tBuOK/tBuOH EtOH KOH 州 EtOH tBuOK BuOH preferred for 1°RX
B. Dehydrohalogenation of alkyl halides C H C X + B C C + BH + X strong base: KOH/ethanol CH3CH2ONa/CH3CH2OH tBuOK/tBuOH Br EtONa EtOH Cl KOH EtOH Cl tBuOK tBuOH preferred for 1º RX Elimination
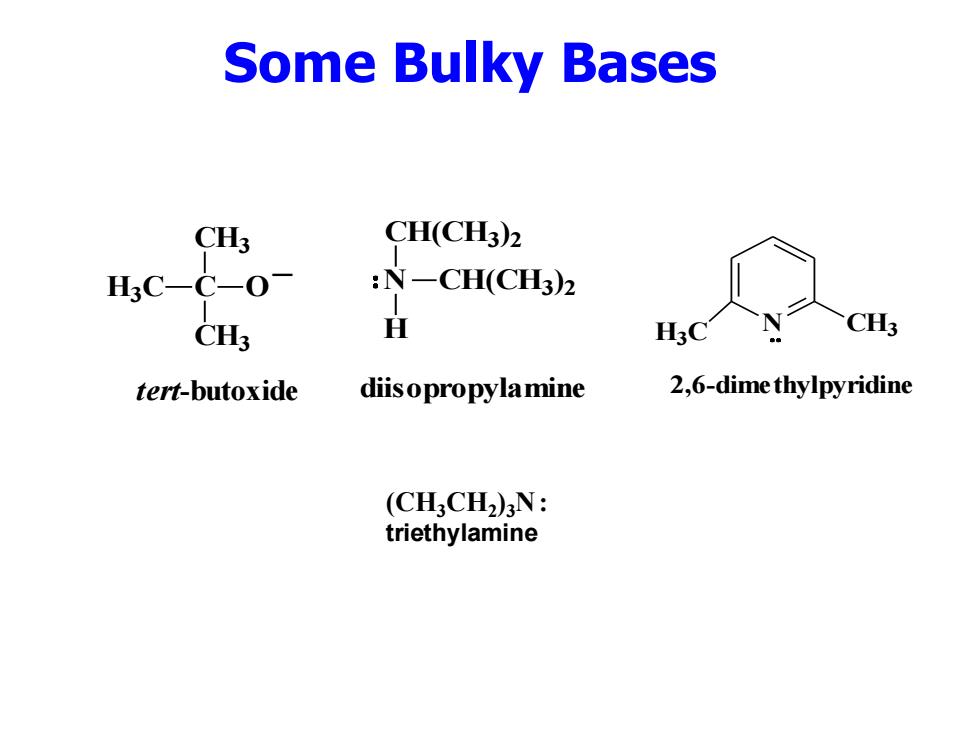
Some Bulky Bases CH3 CH(CH3)2 H3C-C-O- :N一CH(CH3)2 CH3 H CH3 tert-butoxide diisopropylamine 2,6-dime thylpyridine (CHCH2)N: triethylamine
Some Bulky Bases C CH3 H3C CH3 O _ tert-butoxide (CH3CH2 )3N : triethylamine N H CH(CH3 ) 2 CH(CH3 ) 2 diisopropylamine H N CH3 3C 2,6-dimethylpyridine