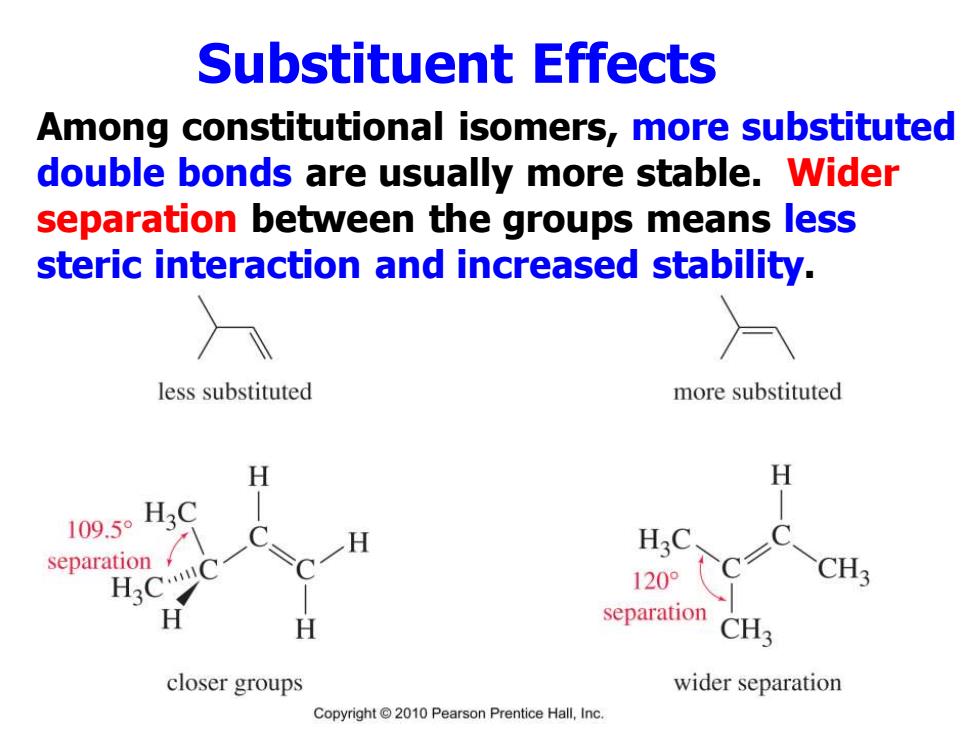
Substituent Effects Among constitutional isomers,more substituted double bonds are usually more stable.Wider separation between the groups means less steric interaction and increased stability. less substituted more substituted H H 109.50H3 separation 120° CH3 separation H CH3 closer groups wider separation Copyright 2010 Pearson Prentice Hall,Inc
Substituent Effects Among constitutional isomers, more substituted double bonds are usually more stable. Wider separation between the groups means less steric interaction and increased stability

Disubstituted Isomers trans more stable than cis cis geminal trans isomer Less stable isomer is higher in energy,has a more exothermic heat of hydrogenation. Cis-2-butene Bc=cC内 -120kJ H Isobutylene (CH3)2C=CH2 -117kJ Trans-2-butene HC-C CH3 -116kJ CHg -H steric repulsion
Disubstituted Isomers ❖trans more stable than cis ◼cis < geminal < trans isomer ❖Less stable isomer is higher in energy, has a more exothermic heat of hydrogenation. Trans-2-butene -116 kJ Isobutylene (CH3)2C=CH2 -117 kJ Cis-2-butene CH3 -120 kJ C C CH3 H H H C C CH3 CH3 H steric repulsion C C H H H H H H H H C C H H H H H H H H

Substituent Effects More substituted alkenes are more stable. H,C=CH2<R-CH=CH2<R-CH=CH-R< R-CH=CR2<R2C=CR2 unsub.monosub.disub.< trisub.tetra sub. Alkyl group stabilizes the double bond. Alkene less sterically hindered
Substituent Effects ❖More substituted alkenes are more stable. H2C=CH2 < R-CH=CH2 < R-CH=CH-R < R-CH=CR2 < R2C=CR2 ❖unsub. < monosub. < disub. < trisub. < tetra sub. ❖Alkyl group stabilizes the double bond. ❖Alkene less sterically hindered

Relative Stabilities ethylene, unsubstituted 2.7 keal (11k monosubstituted 用 C=C 4.2 kcal (18kJ K3ou3 4.8 kcal (20kJ 5.2 kcal (22kJ) 5.9 kcal disubstituted geminal 6.2 kcal H trans (25kJ R H (26kJ C=C trisubstituted R tetrasubstituted R R
Relative Stabilities

Stability of Cycloalkene ring connects CH, CH,H CH,H CH2-CH2 H behind the double bond. CH, CH2 CH2 CH CH, H CH2 H CH, H CH2 CH,-CH2 H trans cyclic system trans-cycloheptene trans-cyclooctene cis-cyclooctene marginally stable stable more stable Copyright2010 Pearson Prentice Hall,Inc. Cis isomer more stable Small rings have additional ring strain. Must have at least eight carbons to form a stable trans double bond. For cyclodecene (and larger),the trans double bond is almost as stable as the cis
Stability of Cycloalkene ❖Cis isomer more stable ❖Small rings have additional ring strain. ❖Must have at least eight carbons to form a stable trans double bond. ❖For cyclodecene (and larger), the trans double bond is almost as stable as the cis