
12.3.5 DecarboxylationCH;COONa + NaOH 热熔,→CH4↑ + Na2CO3HOOCCH.COOHCH3COOH + CO2CH,COCH,COOHCH.COCH+COCI3CCOOHCHCl3+ CO2干CC14C6HsCH2COOAg + Br2CHsCH2Br+CO2+AgBr76℃反应Hunsdiecker
12.3.5 Decarboxylation Hunsdiecker 反应 CH3 COONa + NaOH 热熔 CH4 + Na2 CO3 HOOCCH2 COOH CH3 COOH + CO2 CH3 COCH2 COOH CH3 COCH3 + CO2 Cl 3 CCOOH CHCl 3 + CO2 C6 H5 CH2 COOAg + Br 2 干 CCl 4 C6 H5 CH2 Br + CO2 + AgBr 76 o C

12.3.6 Halogenation of a-HP或PBr3CH,CH,CH,CH,CHCOOH + HBrCH3(CH2)4COOH+Br2BrHell-Volhard-Zelinsky reaction
12.3.6 Halogenation of α-H CH3 (CH2 ) 4 COOH + Br 2 P 或 PBr 3 CH3 CH2 CH2 CH2 CHCOOH + HBr Br ——Hell-Volhard-Zelinsky reaction
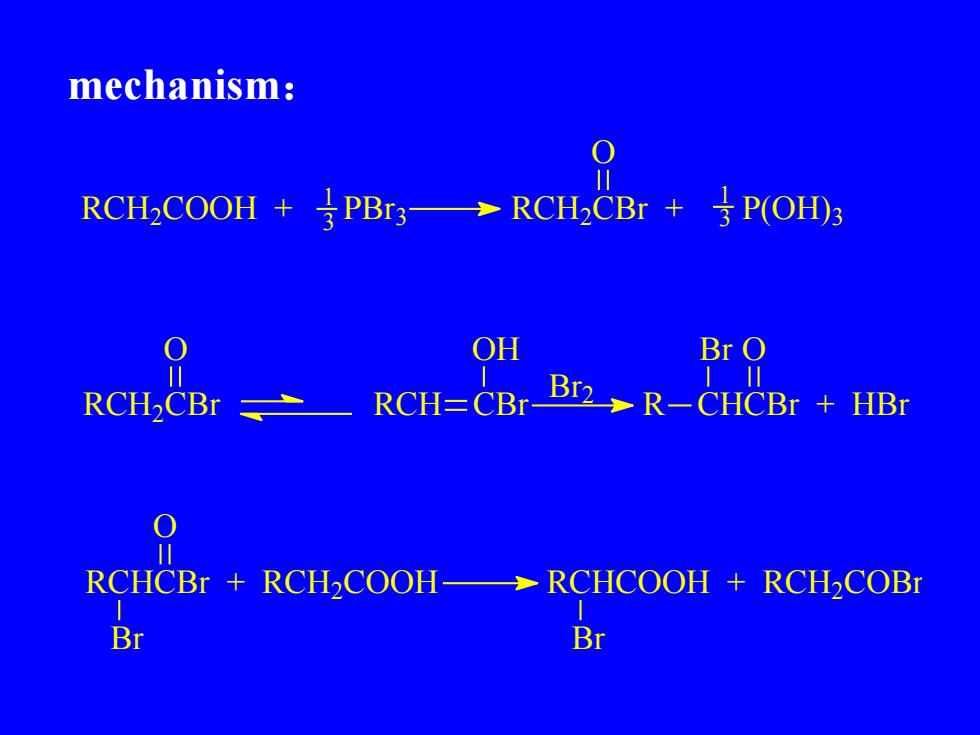
mechanism:11↓ P(OH)3RCH.COOH+PBr3RCH,CBr +一OHBr O01Br2_R-CHCBr + HBrRCH=CBrRCH,CBrO1RCHCBr + RCH,COOHRCHCOOH + RCHCOB11BrBr
mechanism: RCH2 COOH + 3 PBr 3 1 RCH2 CBr + P(OH) 3 O 3 1 RCH2 CBr O RCH CBr OH Br 2 R CHCBr + HBr Br O RCHCBr + RCH2 COOH Br O RCHCOOH + RCH2 COBr Br
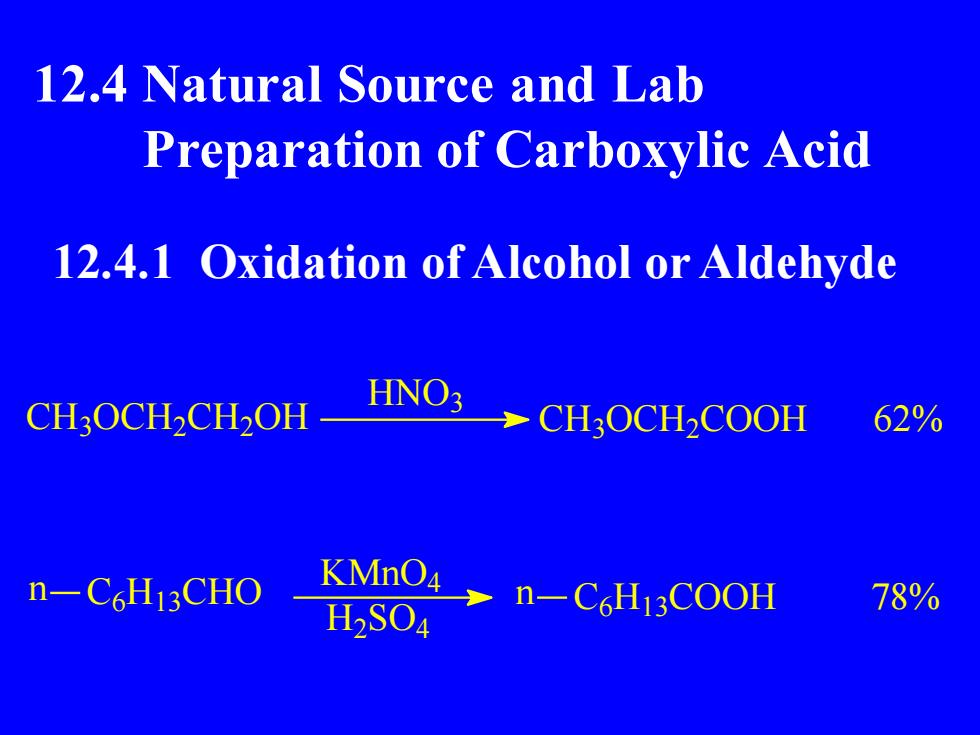
12.4 Natural Source and LabPreparation of Carboxylic Acid12.4.1 Oxidation ofAlcohol or AldehydeHNO362%CH3OCH,CH2OHCH:OCH,COOHKMnO4n-C6Hi3CHO78%n-C6H13COOHH2SO4
12.4 Natural Source and Lab Preparation of Carboxylic Acid 12.4.1 Oxidation of Alcohol or Aldehyde CH3 OCH2 CH2 OH HNO3 CH3 OCH2 COOH 62% n C6 H13 CHO KMnO4 H2 SO4 n C6 H13 COOH 78%
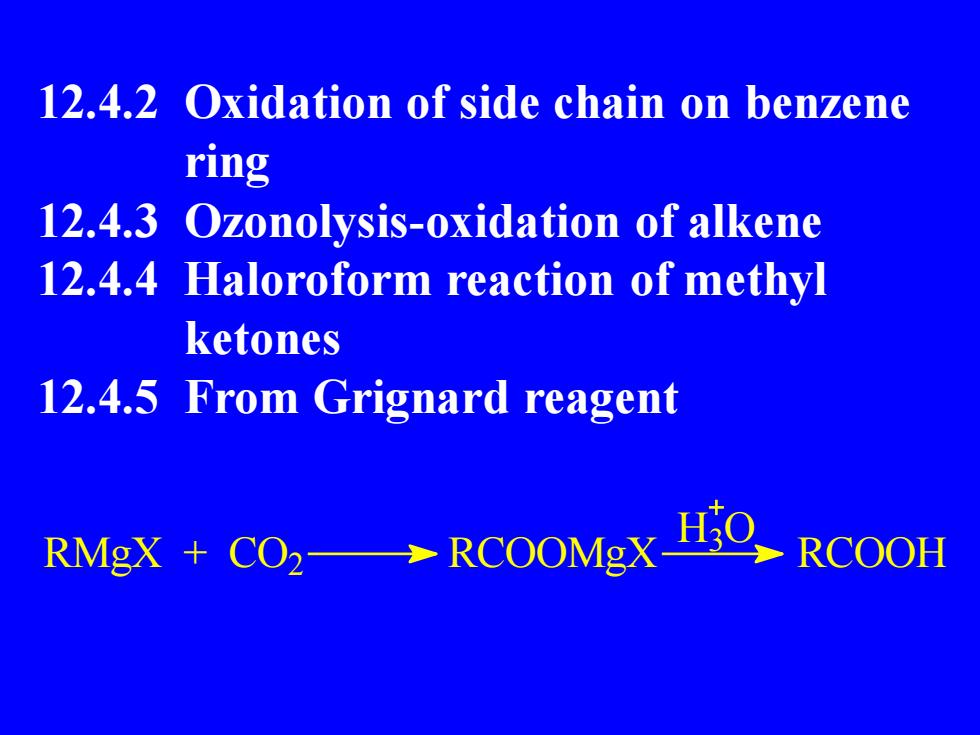
12.4.2 Oxidation of side chain on benzenering12.4.3 Ozonolysis-oxidation of alkene12.4.4 Haloroform reaction of methylketones12.4.5 From Grignard reagentH.ORMgX + CO2→RCOOMgXRCOOH
12.4.2 Oxidation of side chain on benzene ring 12.4.3 Ozonolysis-oxidation of alkene 12.4.4 Haloroform reaction of methyl ketones 12.4.5 From Grignard reagent RMgX + CO2 RCOOMgX RCOOH H3 O