
INTRODUCTION 3 polymer can be made.Other efforts related to polymeric waste have focused on reducing the seemingly infinite lifetime of many plastics in the environment by developing biodegradable commodity polymers. There are five major areas of application for polymers:(1)plastics,(2)rubbers or elastomers.(3)fibers,(4)surface finishes and protective coatings,and (5)adhesives. Despite the fact that all five applications are based on polymers,and in many cases the same polymer is used in two or more,the industries pretty much grew up separately.It was only after Dr.Harmann Staudinger [1,2]proposed the "macromolecular hypothesis"in the 1920s explaining the common molecular makeup of these materials(for which he won the 1953 Nobel Prize in chemistry in belated recognition of the importance of his work)that polymer science began to evolve from the independent technologies.Thus,a sound fundamental basis was established for continued technological advances.The history of polymer science is treated in detail elsewhere [3,4]. Economic considerations alone would be sufficient to justify the impressive scientific and technological efforts expended on polymers in the past several decades.In addition, however,this class of materials possesses many interesting and useful properties complete- ly different from those of the more traditional engineering materials and that cannot be explained or handled in design situations by the traditional approaches.A description of three simple experiments should make this obvious. 1.Silly putty,a silicone polymer,bounces like rubber when rolled into a ball and dropped.On the other hand,if the ball is placed on a table,it will gradually spread to a puddle.The material behaves as an elastic solid under certain conditions and as a viscous liquid under others. 2.If a weight is suspended from a rubber band,and the band is then heated(taking care not to burn it),the rubber band will contract appreciably.All materials other than polymers will undergo thermal expansion upon heating (assuming that no phase transformation has occurred over the temperature range). 3.When a rotating rod is immersed in a molten polymer or a fairly concentrated polymer solution,the liquid will actually climb up the rod.This phenomenon,the Weissenberg effect,is contrary to what is observed in nonpolymer liquids,which develop a curved surface profile with a lowest point at the rod,as the material is flung outward by centrifugal force. Although such behavior is unusual in terms of the more familiar materials,it is a perfectly logical consequence of the molecular structure of polymers.This molecular structure is the key to an understanding of the science and technology of polymers and will underlie the chapters to follow. Figure 1.1 illustrates the followings questions to be considered: 1.How is the desired molecular structure obtained? 2.How do the polymer's processing (i.e..formability)properties depend on its molecular structure? 3.How do its material properties (mechanical,chemical,optical,etc.)depend on molecular structure? 4.How do material properties depend on a polymer's processing history? 5.How do its applications depend on its material properties?

INTRODUCTION (2) Processing Molecular structure conditions (1) Polymerization (3) (4) (synthesis) Material properties (5) Applications FIGURE 1.1 The key role of molecular structure in polymer science and technology The word polymer comes from the Greek word meaning "many-membered."Strictly speaking.it could be applied to any large molecule formed from a relatively large number of smaller units or"mers,"for example,a sodium chloride crystal,but it is most commonly (and exclusively.here)restricted to materials in which the mers are held together by covalent bonding,that is,shared electrons.For our purposes,only a few bond valences need be remembered: -c- -0-ClF-H- It is always a good idea to "count the bonds"in any structure written to make sure they conform to the above. Example 1.I Carbon is the most common element in polymers.Why? Solution.To make a polymer,the atoms that make up the repeat units must be capable of forming at least two bonds,so H,F,and Cl would terminate a polymer,and can be only on the ends or in functional groups on the sides of the polymer.Two bonds are enough for bonding to occur to continue a polymer structure,but using only oxygen(O-0-0...) would not provide any functionality.C and Si both can make four bonds,and since most polymers are derived from petroleum sources,carbon is found in the vast majority of polymers.The four bonds allow two to be used to form the backbone(C-C-C...),but the remaining two can be used to include different functional groups,which give polymers their unique characteristics. The basic structure of a polymer consists of a backbone and pendant (or side)groups (Figure 1.2).Atoms that are covalently linked and stretch from one end of a polymer to the other make up the polymer backbone(which is often not only carbon,but can also contain other atoms such as N.O.or Si).All other atoms are part of the side groups(H is the simplest,methyl(-CH3)or alcohol(-OH)groups are among many possibilities,making for a wide variety of structures that make up polymers).Polymers are named based on the repeat unit since that can describe a rather lengthy molecule in a succinct way. Because carbon is so versatile and can form the backbone of a polymer while being covalently attached to two other side groups,some basic organic chemistry will help to understand the molecular structure of polymers.While organic chemistry is not a prerequisite for learning about polymers,some basic terminology related to organic functional groups will help in understanding molecular structures and polymerization reactions

INTRODUCTION 5 Polypropylene A segment of polypropylene with repeat unit five repeat units drawn out 一(CH-CH)- HH HH HHHHHH CH3 -(C-C-C-C-C一C一C一C一C一C)- H CH:H CH3H CH:H CH:H CH3 FIGURE 1.2 Structure of a typical polymer polypropylene is shown here,with an all-carbon backbone and side groups of-H or-CH3.Note that fixed bond angles make the three-dimensional structure more complex than shown here. Organic chemicals can be categorized based on common functional groups(types of bonds)that are found within a structure(Figure 1.3).These functionalities are helpful in determining if a molecule might be reactive,whether it is likely to be hydrophilic (or hydrophobic).and how (or if)a molecule can participate in a polymerization reaction. Alcohols(-OH)are perhaps one of the more familiar functional groups,and they can be reacted with carboxylic acids(-COOH)to form an ester bond.As long as each molecule has at least two reactive functional groups (e.g.,a dialcohol (more commonly referred to as a diol)and a dicarboxylic acid (a diacid)can react to form a continuous polymer molecule with multiple ester linkages along the polymer backbone,yielding a polyester!).Alcohols can also be found in the side groups of polymers,as in poly(vinyl alcohol),where the-OH group stays intact after the reaction. Polyester:two repeat units Poly(vinyl alcohol):two repeat units 0 0 --CH2-CH-CH2-CH于 千(CH24CO-(CH24C-O-} OH OH Polyester backbone atoms Poly(vinyl alcohol)backbone atoms C-C-C-C-C-O-C-C-C-C-C-O C-C-C-C Note that the hydrogens written alongside carbons are not part of the backbone of a polymer molecule.They can bond only to one other atom (the carbon),so a more accurate drawing would show the hydrogens off to the side of the backbone,just as the-OH group is in the poly(vinyl alcohol). Some of the organic functional groups that are reactive to form polymers include alcohols,amines,carboxylic acids,and alkenes.Although alkanes (C-C bonds)are common in polymers,both in the backbone and in the side groups,they are not reactive. Other bonds commonly found in polymers include amides(for nylons),esters,carbonates, imides,and urethanes.Of this last set,even those who have had organic chemistry probably have not run across the last three,as they are more common in polymers than in traditional organic chemistry.Further reviews of organic chemistry functional groups are also available,and many others can be found online [5,6]. While there is no simple rule about the structures found in a polymer,the most common polymers have only a few organic functional groups,that repeat over and over to make the macromolecules.Of the groups shown in Figure 1.3,alkanes,esters,urethanes,carbonates, aryls,silanes,amides,and imides are commonly found in the backbone of polymers. Alcohols,carboxylic acids,and amines are either reactive parts of monomers or can be found in polymer side groups
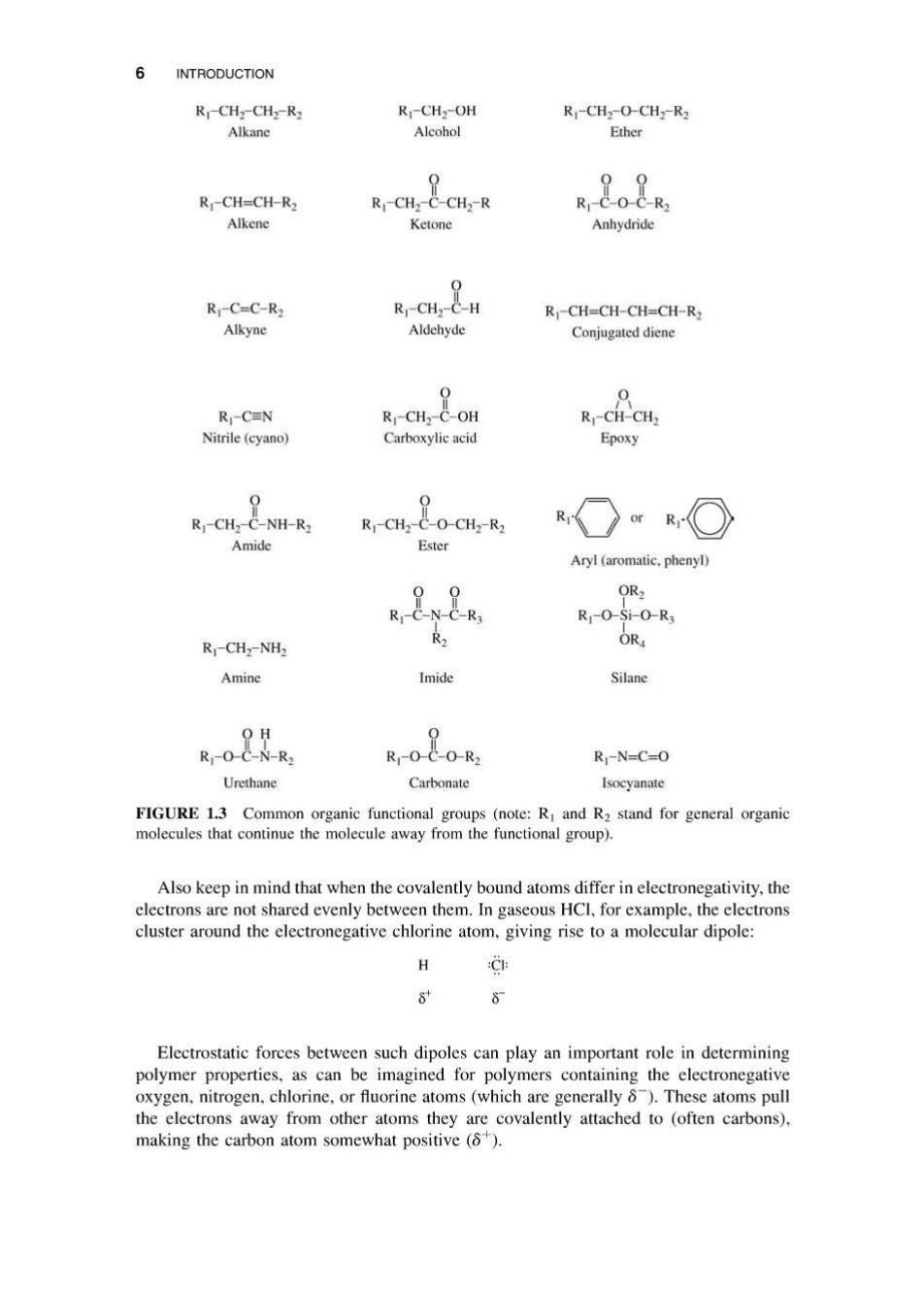
6 INTRODUCTION R-CHz-CHz-R2 Rj-CH2-OH R-CHz-O-CH2-R2 Alkane Alcohol Ether 09 R-CH=CH-R2 R-CH2-C-CHz-R Rj-C-O-C-R2 Alkene Ketone Anhydride R-C=C-R2 R-CH2-C-H Rj-CH=CH-CH=CH-R2 Alkyne Aldehyde Conjugated diene 0 R-CEN R-CH2-C-OH R-CH-CH2 Nitrile(cyano) Carboxylic acid Epoxy 0 Rj-CH2-C-NH-R2 Rj-CH2-C-O-CH2-R2 Amide Ester Aryl (aromatic.phenyl) OR2 R-C-N-C-R3 Rj-O-Si-O-R3 R Rj-CHz-NH2 OR Amine Imide Silane OH 9 Rj-O-C-N-R2 R1-0-C-O-R2 R-N=C=0 Urethane Carbonate Isocyanate FIGURE 1.3 Common organic functional groups (note:R and R2 stand for general organic molecules that continue the molecule away from the functional group). Also keep in mind that when the covalently bound atoms differ in electronegativity,the electrons are not shared evenly between them.In gaseous HCl,for example,the electrons cluster around the electronegative chlorine atom,giving rise to a molecular dipole: H CE 8 8 Electrostatic forces between such dipoles can play an important role in determining polymer properties,as can be imagined for polymers containing the electronegative oxygen,nitrogen.chlorine,or fluorine atoms(which are generally 8).These atoms pull the electrons away from other atoms they are covalently attached to (often carbons), making the carbon atom somewhat positive(6)

REFERENCES 7 The most important constituents of living otmanisms,cellulose and proteins,are naturally occurring polymers (as is DNA),but most commercially important polymers are synthetic or modified natural polymers. PROBLEMS 1 Consider the room you are in while doing your homework (dorm room,library, cafeteria.pub..and so on). (a)Identify five items in it that are made of polymers. (b)What would you make those items of if there were no polymers? (c)Why do you suppose polymers were chosen over competing materials (if any) for each particular application? 2 Consider the plastic used in the construction of an automobile.List four different parts of a car that may be made of a polymer(consider the exterior,under the hood, and interior of the car-there are many more than four!).For each part,list two physical or chemical properties important for that part.For example,for plastic cup holders,the polymer must be moldable to form into correct shape and should not turn rubbery when heated to ~150F(if exposed to the sun or a hot cup of coffee). 3 You wish to develop a polymer to replace glass in window glazing.What properties must a polymer have for that application? 4 Vinyl chloride is the monomer from which the commercially important polymer poly (vinyl chloride),PVC,is made.It has the chemical formula C2H3Cl.Show the structure of vinyl chloride and identify any dipoles present. 5 Acrylonitrile monomer.C3H3N,is an important constituent of acrylic fibers and nitrile rubber.It does not have a cyclic structure and it has only one double bond. (a)Show the structure of acrylonitrile and identify any dipoles present. (b)Draw the repeating structure of polyacrylonitrile,ignore end groups. 6 Poly(octyl cyanoacrylate)is an important adhesive.With the help of the Internet,draw the repeating structure for this polymer,show any dipoles present,and find one trademarked product made from poly(octyl cyanoacrylate). 7 Propylene(C3H6)is the monomer from which the fastest growing plastic,polypropylene. is made.It contains one double bond.Show its structure and identify any dipoles present. REFERENCES [1]Staudinger,H..Ber.Disch.Chem.Ges.53,1073 (1920). [2]Staudinger,H.and J.Fritsch.Helv.Chim.Acta 5,778(1922) [3]Morawetz,H..Polymers:The Origins and Growth ofa Science,Wiley-Interscience,New York,1985. [4]Stahl,G.A..CHEMTECH,August 1984,492. [5]Richardson,P.N.and R.C.Kierstead,SPE J.25(9).54 (1969). [6]Leach,M.R..Organic Functional Groups,on website Chemistry Tutorials and Drills,www. chemistry-drills.com/functional-groups.php?q=simple,2009