
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 13 Nuclear Magnetic Resonance Spectroscopy Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 13 Nuclear Magnetic Resonance Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr
Introduction NMR is the most powerful tool available for organic structure determination. It is used to study a wide variety of nuclei: X1H >13C >15N >19F >31P => Chapter 13 2
Chapter 13 2 Introduction • NMR is the most powerful tool available for organic structure determination. • It is used to study a wide variety of nuclei: ➢1H ➢13C ➢15N ➢19F ➢31P =>
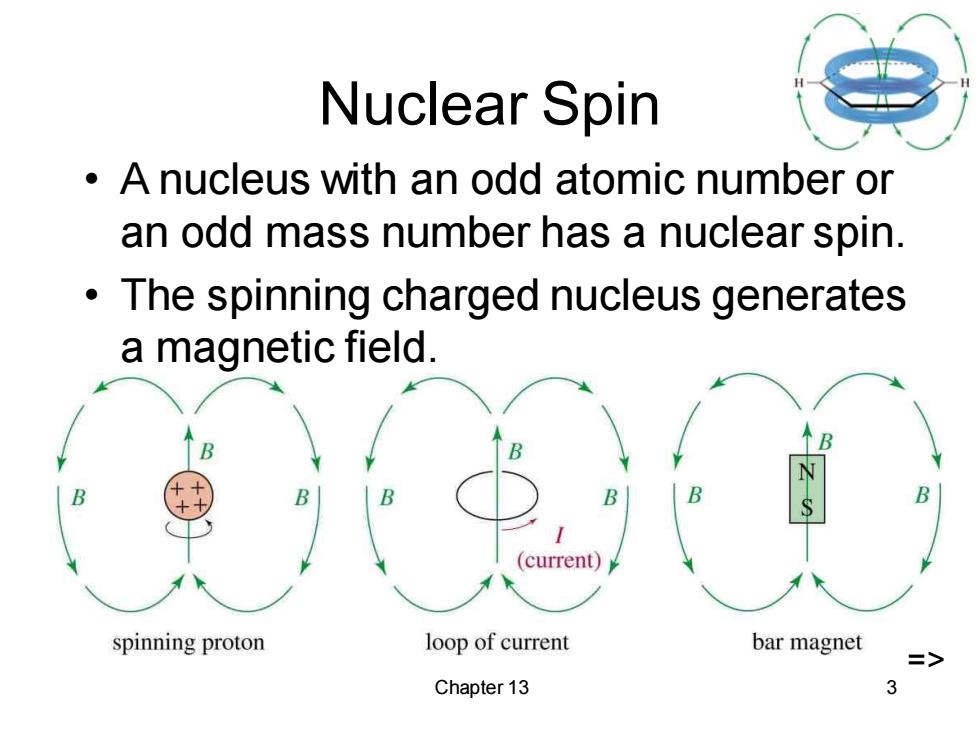
Nuclear Spin A nucleus with an odd atomic number or an odd mass number has a nuclear spin. The spinning charged nucleus generates a magnetic field. (current) spinning proton loop of current bar magnet => Chapter 13 3
Chapter 13 3 Nuclear Spin • A nucleus with an odd atomic number or an odd mass number has a nuclear spin. • The spinning charged nucleus generates a magnetic field. =>
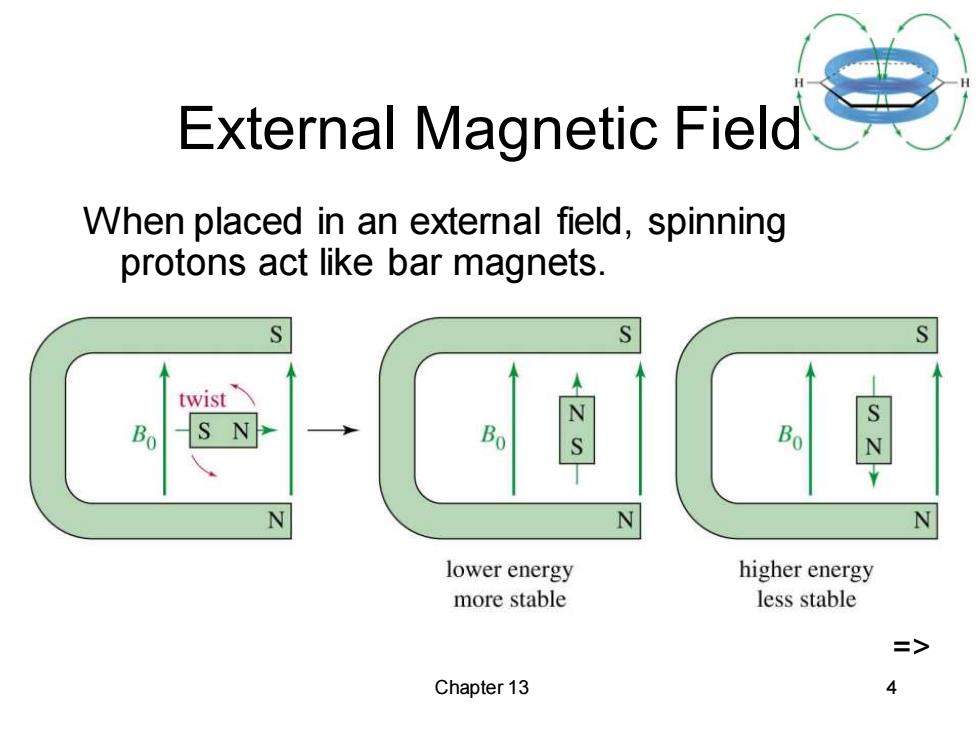
External Magnetic Field When placed in an external field,spinning protons act like bar magnets. S twist N S N 60 Bo N N N N lower energy higher energy more stable less stable 三> Chapter 13 4
Chapter 13 4 External Magnetic Field When placed in an external field, spinning protons act like bar magnets. =>
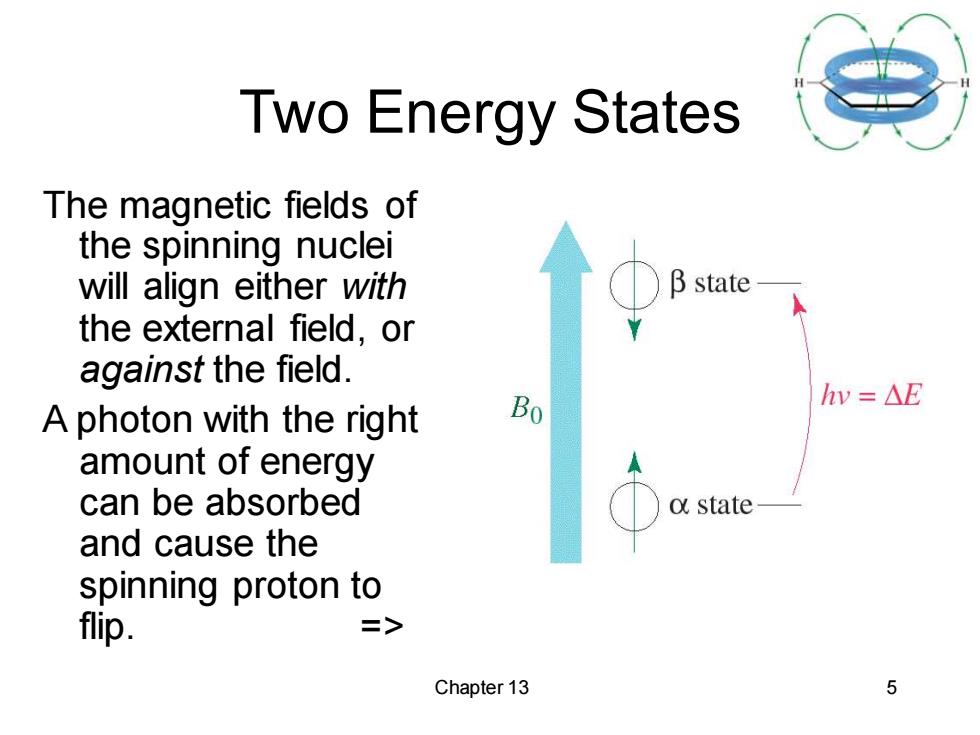
Two Energy States The magnetic fields of the spinning nuclei will align either with B state the external field,or against the field. Bo hv=△E A photon with the right amount of energy can be absorbed o state and cause the spinning proton to flip. => Chapter 13 5
Chapter 13 5 Two Energy States The magnetic fields of the spinning nuclei will align either with the external field, or against the field. A photon with the right amount of energy can be absorbed and cause the spinning proton to flip. =>