
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 5 Stereochemistry Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 5 Stereochemistry Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr
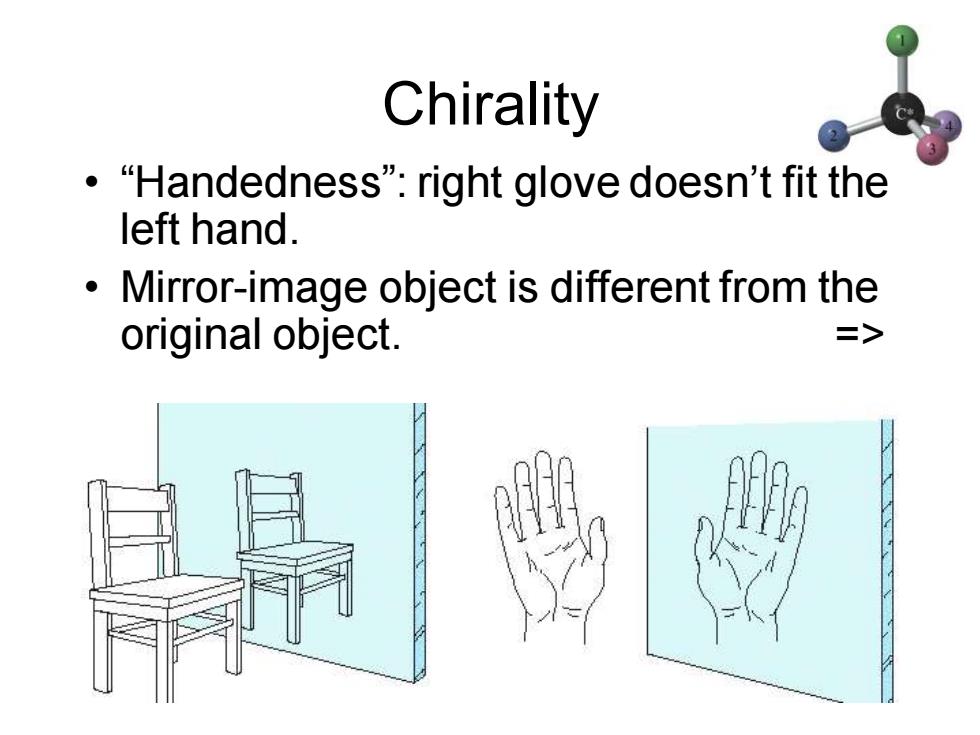
Chirality ·“Handedness"”:right glove doesn't fit the left hand. Mirror-image object is different from the original object. =>
Chapter 5 2 Chirality • “Handedness”: right glove doesn’t fit the left hand. • Mirror-image object is different from the original object. =>
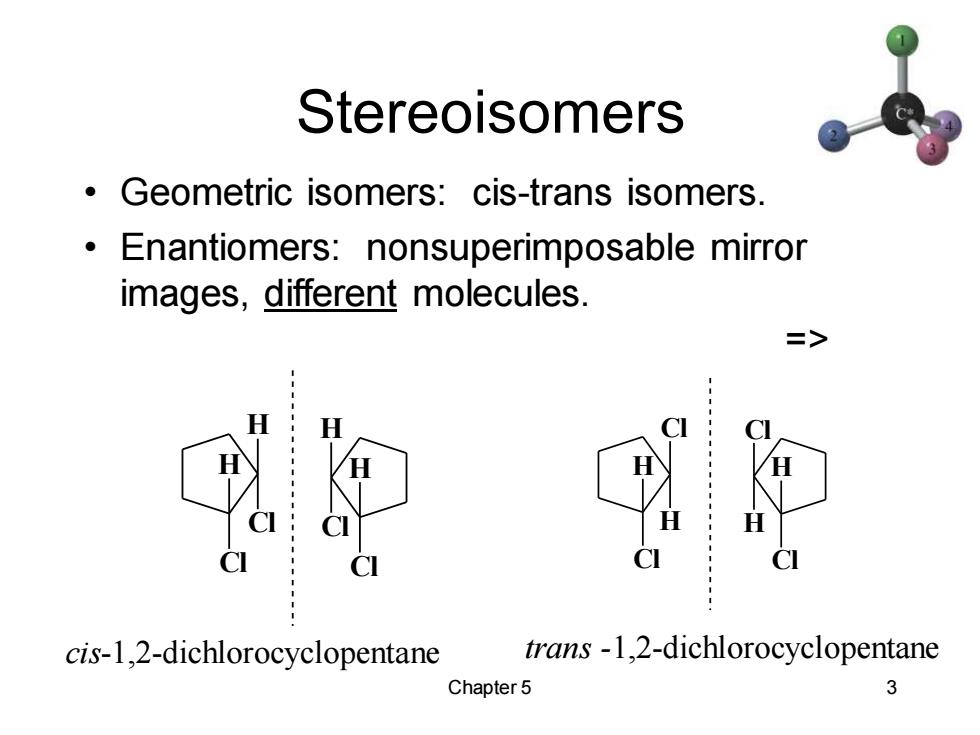
Stereoisomers Geometric isomers:cis-trans isomers. Enantiomers:nonsuperimposable mirror images,different molecules. => cis-1,2-dichlorocyclopentane trans-1,2-dichlorocyclopentane Chapter 5 3
Chapter 5 3 Stereoisomers • Geometric isomers: cis-trans isomers. • Enantiomers: nonsuperimposable mirror images, different molecules. => cis-1,2-dichlorocyclopentane trans -1,2-dichlorocyclopentane H Cl H Cl H Cl H Cl H Cl Cl H H Cl Cl H
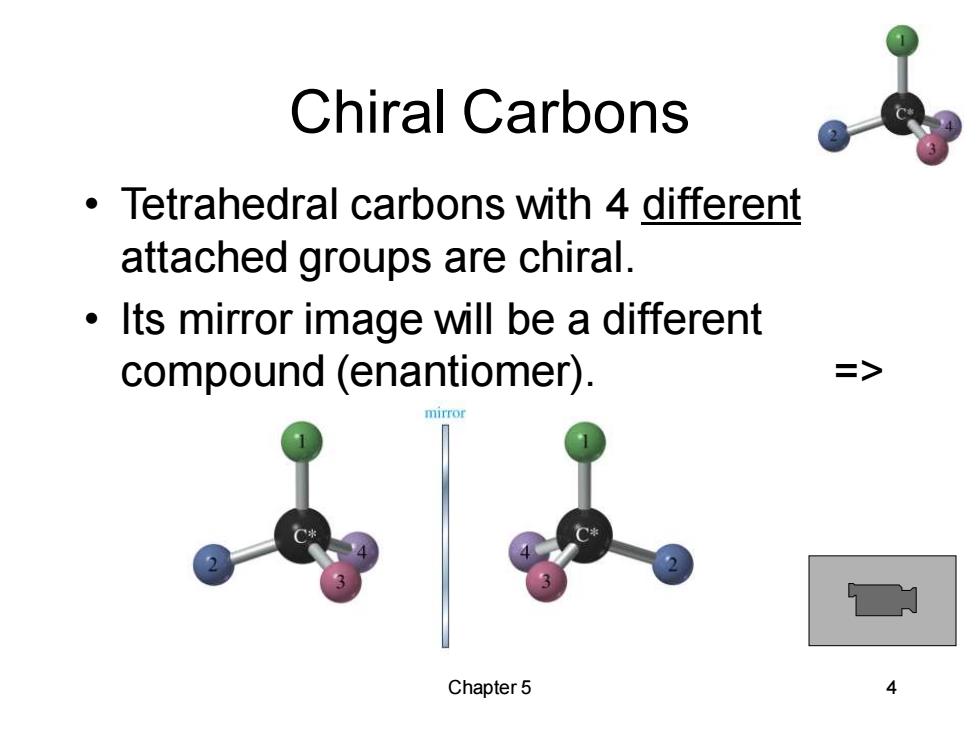
Chiral Carbons Tetrahedral carbons with 4 different attached groups are chiral. Its mirror image will be a different compound (enantiomer). > Chapter5
Chapter 5 4 Chiral Carbons • Tetrahedral carbons with 4 different attached groups are chiral. • Its mirror image will be a different compound (enantiomer). =>
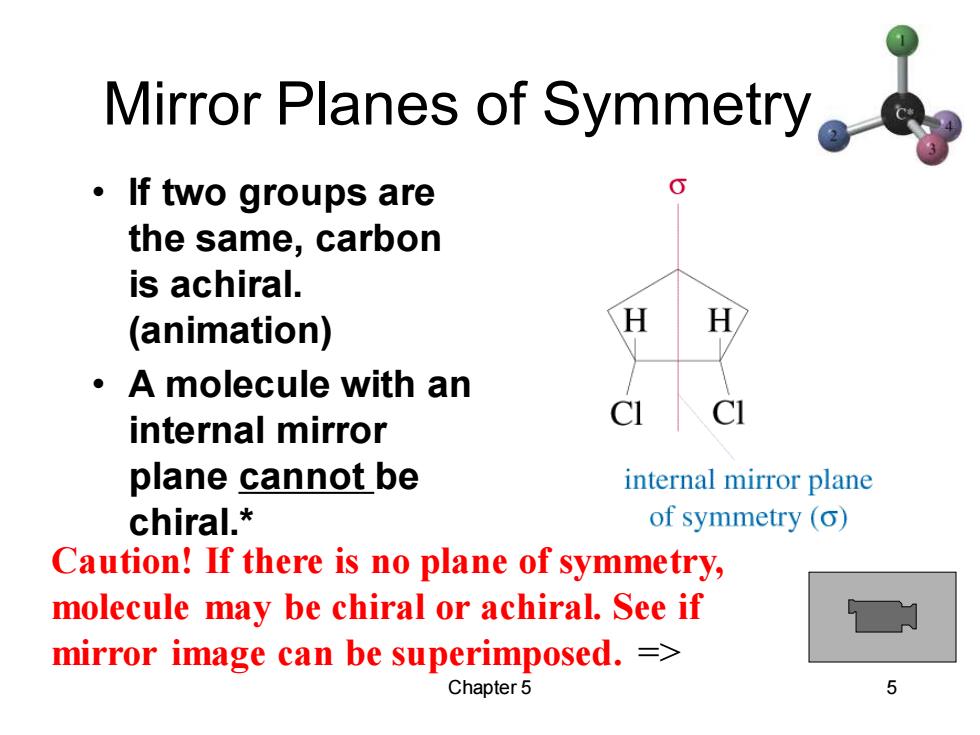
Mirror Planes of Symmetry ·If two groups are the same,carbon is achiral. (animation) 。A molecule with an C 1 internal mirror plane cannot be internal mirror plane chiral.* of symmetry (o) Caution!If there is no plane of symmetry, molecule may be chiral or achiral.See if mirror image can be superimposed.= Chapter 5 5
Chapter 5 5 Mirror Planes of Symmetry • If two groups are the same, carbon is achiral. (animation) • A molecule with an internal mirror plane cannot be chiral.* Caution! If there is no plane of symmetry, molecule may be chiral or achiral. See if mirror image can be superimposed. =>