
Organic Chemistry,5th Edition L.G.Wade,Jr. Chapter 10 Structure and Synthesis of Alcohols Jo Blackburn Richland College,Dallas,TX Dallas County Community College District ©2003,Prentice Hall
Chapter 10 Structure and Synthesis of Alcohols Jo Blackburn Richland College, Dallas, TX Dallas County Community College District © 2003, Prentice Hall Organic Chemistry, 5th Edition L. G. Wade, Jr
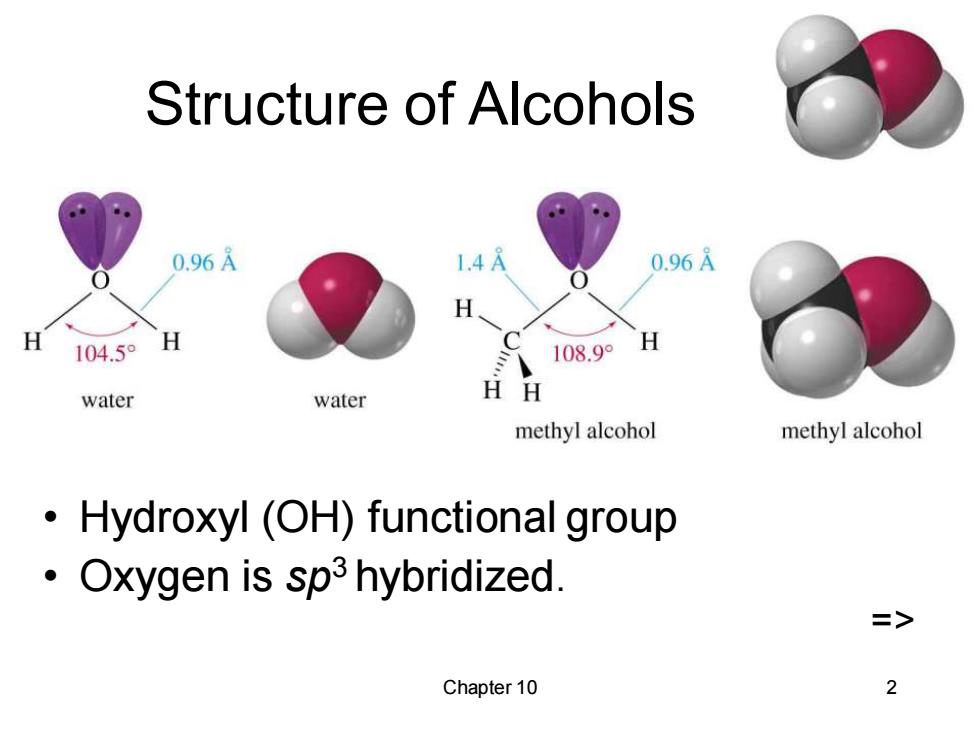
Structure of Alcohols 0.96A .4 0.96A H 104.50 H 108.9° H water water HH methyl alcohol methyl alcohol Hydroxyl (OH)functional group Oxygen is sp3 hybridized. Chapter 10 2
Chapter 10 2 Structure of Alcohols • Hydroxyl (OH) functional group • Oxygen is sp3 hybridized. =>
Classification Primary:carbon with -OH is bonded to one other carbon. Secondary:carbon with -OH is bonded to two other carbons. Tertiary:carbon with-OH is bonded to three other carbons. Aromatic (phenol):-OH is bonded to a benzene ring. => Chapter 10 3
Chapter 10 3 Classification • Primary: carbon with –OH is bonded to one other carbon. • Secondary: carbon with –OH is bonded to two other carbons. • Tertiary: carbon with –OH is bonded to three other carbons. • Aromatic (phenol): -OH is bonded to a benzene ring. =>
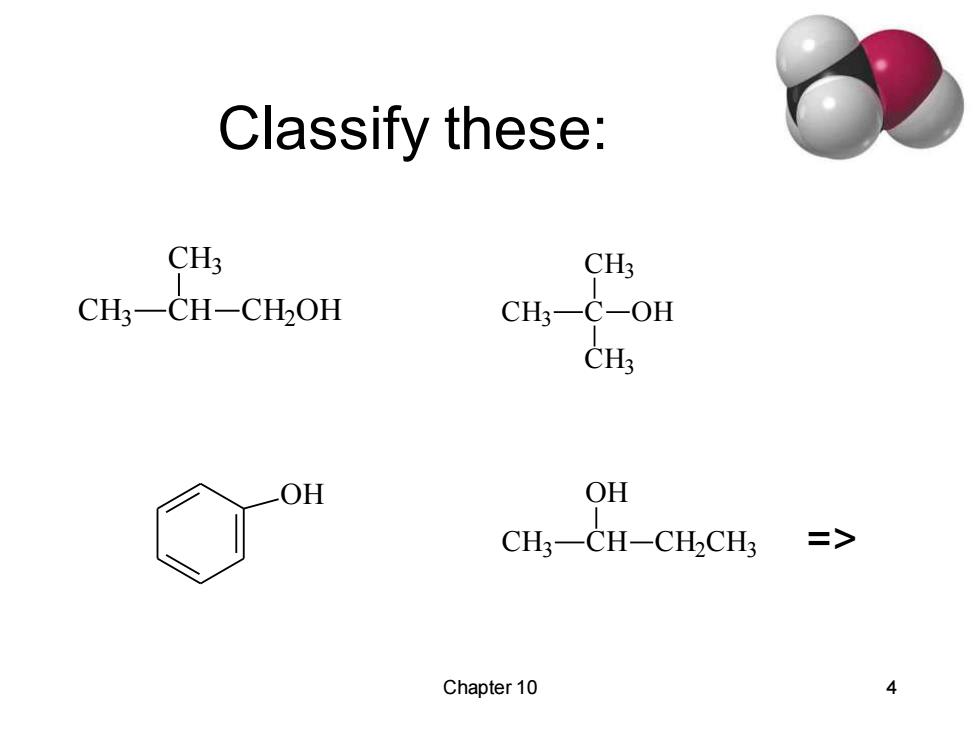
Classify these: CH3 CH3 CH3一CH-CHOH CH3-C-OH OH OH CH3-CH-CH2CH3 二> Chapter 10
Chapter 10 4 Classify these: CH3 CH CH3 CH2OH CH3 C CH3 CH3 OH OH CH3 CH OH CH2CH3 =>
IUPAC Nomenclature Find the longest carbon chain containing the carbon with the -OH group. Drop the -e from the alkane name,add- ol. Number the chain,starting from the end closest to the -OH group. Number and name all substituents.= Chapter 10 5
Chapter 10 5 IUPAC Nomenclature • Find the longest carbon chain containing the carbon with the -OH group. • Drop the -e from the alkane name, add - ol. • Number the chain, starting from the end closest to the -OH group. • Number and name all substituents. =>