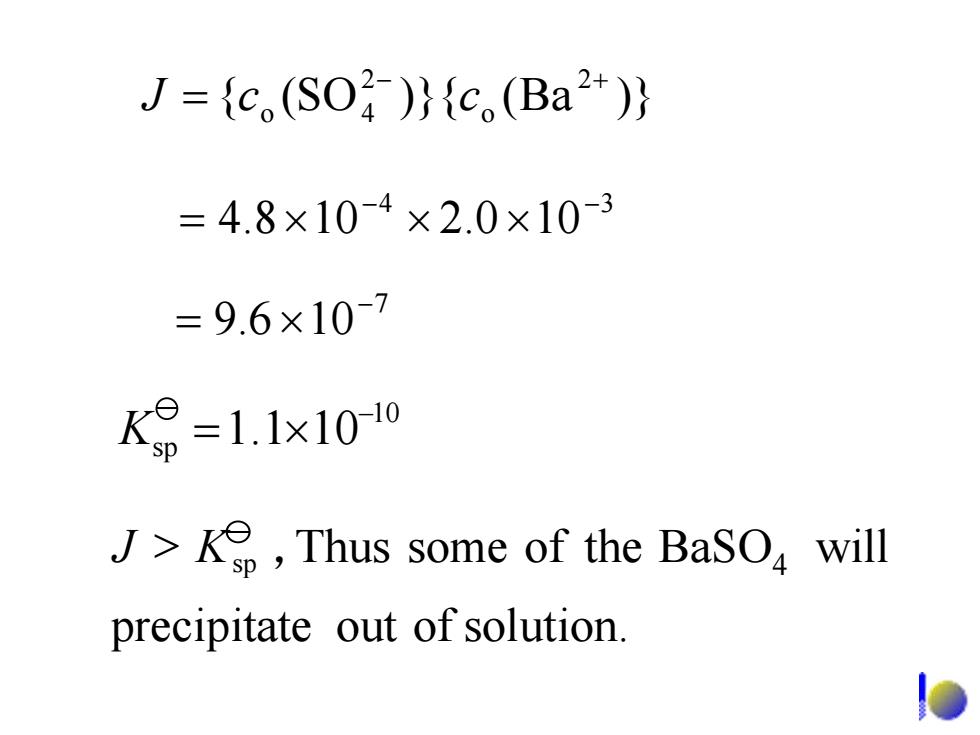
J={c(SO)}{c,(Ba2+)} =4.8×10-4×2.0×10-3 =9.6×10-7 K9=1.1x1010 J>,Thus some of the BaSO will precipitate out of solution. lo
7 106.9 − ×= 4 3 100.2108.4 − − ×××= )}Ba()}{SO({ 2 o 24o − + = cJ c 10 sp 101.1 − K ×= precipitate out of solution. BaSO will 4 > KJ sp , someThus of the
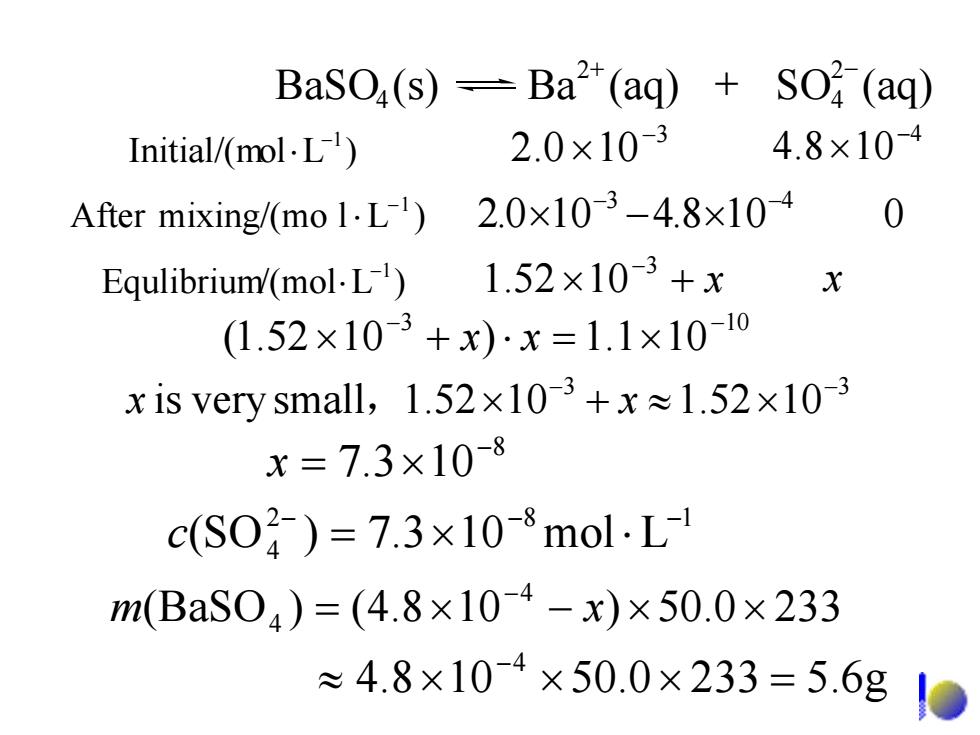
BaSO(s)-Ba2(aq)+S(aq) Initial/(mol.L) 2.0×10-3 4.8×10-4 After mixing/(mo 1.L)2.0x10-3-4.8x10-4 0 Equlibrium/(mol.L)1.52x10-3+x X (1.52×103+x)x=1.1×10-10 x is very small,.1.52×103+x≈1.52×10-3 x=7.3×10-8 c(S04)=7.3×10-8molL m(BaS04)=(4.8×104-x)×50.0×233 ≈4.8×104×50.0×233=5.6g0
3 100.2 − × 4 108.4 − )LolInitial/(m × −1 ⋅ 3 10 101.1)1052.1( − − xx ×=⋅+× 3 3 1052.11052.1 small very is − − x , x ×≈+× 3 4 108.4100.2 − − After mixing/(mo )Ll ×−× 0 −1 ⋅ +× x −3 Equlibrium )L/(mol 1052.1 x −1 ⋅ BaSO (s) Ba (aq) SO (aq) 24 2 4 + − + 5.6g2330.50108.4 4 =×××≈ − 2330.50)108.4()(BaSO 4 4 ××−×= − m x 2 8 1 4 Lmol103.7)SO( − − − c ⋅×= 8 103.7 − x ×=
6.2.2 The common ion effect and salt effect Common lon Effect 1.The common ion effect The common ion effect is the reduction in the solubility of a sparingly soluble salt by the addition of a soluble salt that has an ion in common with it. 0.010.030.050.07 c(NaF)/(mol-L) CaF,在NaF溶液中的同离子效应 ①-@-⊙⊙-⊙
6.2.2 The common ion effect and salt effect 1.The common ion effect The common ion effect is the reduction in the solubility of a sparingly soluble salt by the addition of a soluble salt that has an ion in common with it