
USDA/FSIS Microbiology Laboratory Guidebook 3rd Edition/1998 decimal into tubes of in triplicate geginning with sted m gassing Follow Use tubes or bottles found to to calculate MPN g Inoubate gassed Owik Seal bags containing botties or tubes at 3710C,shaking at100 rpm for 4 h. After the 4 h incubation additional sterile solution to bring the fat88e ntration in each enrichment vessel to 30 mg/L 42±1.0c a Cont: sphe an enrichments directly and ,ata.1:100 on 1:50 swirling a swab in the broth and twisting it against the side of the vessel to remove excess liquid. Break off the swab tip into a tube contain ng 9 m 。士 0.18 a ate excess liquid as above. Swab approximately 40% of the MCCI then streak from the swabbed area to h19o1a plate, 1e8 vely,0. ml sterile nt od This plating technique may be used provided isolated colonies result. 9. Incu a。 the plates for drops of a humectant such 月g and place it in swarming growth Campylobacter If no 1 48be1 egof62 24+。 microaerobic conditions can be achieved in the jar by either of the following methods: i. a with a gas mixture of 58 02,103 CO2,and 853 N2. the evacuation-replacement procedure a tota mes assure proper 6-4
USDA/FSIS Microbiology Laboratory Guidebook 3rd Edition/1998 6-4 decimal dilutions into 9 ml tubes of HEB in triplicate. Place all bottles and tubes in anaerobic jars. See step g. for jar gassing methods. Follow incubation steps beginning with step d. below. Use tubes or bottles found to contain confirmed Campylobacter to calculate MPN (refer to appropriate tables). d. Incubate gassed Qwik Seal® bags or anaerobic jars containing bottles or tubes at 37 ± 1.0o C, shaking at 100 rpm for 4 h. e. After the 4 h incubation at 37 ± 1.0o C, aseptically add additional sterile cefoperazone solution to bring the final concentration in each enrichment vessel to 30 mg/L. Reestablish the microaerobic atmosphere and increase the temperature to 42 ± 1.0o C. Continue the incubation for 20 h shaking at 100 rpm. f. Swab/streak enrichments directly and at a 1:100 dilution onto MCCDA plates (for cooked products, a 1:50 dilution may be plated). Prepare the dilution by swirling a swab in the broth and twisting it against the side of the vessel to remove excess liquid. Break off the swab tip into a tube containing 9.9 ml of 0.1% peptone water and vortex. Inoculate the plates by placing a swab into the enrichment or dilution and removing excess liquid as above. Swab approximately 40% of the MCCDA plate, then streak from the swabbed area to yield isolated colonies. Alternatively, 0.1 ml portions of the enrichments or dilutions may be plated by spreading with a sterile bent glass rod. This plating technique may be used provided isolated colonies result. g. Incubate the MCCDA plates at 42 ± 1.0o C for 24 h in an anaerobic jar under microaerobic conditions. Add about 4 drops of a humectant such as glycerol to a filter paper and place it in the jar to diminish typical confluent and swarming growth of Campylobacter. If no growth is achieved after 24 h, reincubate the plates for an additional 24 to 48 h to attempt recovery. The microaerobic conditions can be achieved in the jar by either of the following methods: i. Evacuate the air from a vented anaerobic jar to a partial vacuum of 20 inches of Hg and fill the jar with a gas mixture of 5% O2, 10% CO2, and 85% N2. Repeat the evacuation-replacement procedure a total of three times to assure proper atmospheric conditions

USDA/FSIS Microbiology Laboratory Guidebook 3rd Edition/1998 ii. CampyPak PlusTM (BBL)or Gas Generating Kits for 1a) ma materials】 Keepa away from ame when opening. NOTE: anae:Gas generator the only typeed ifainon vente are pe very economical. cement gassing vented naero jars a is to faciiitate lid by oponingo clapedcecunnaorobac jas,ofb depressing the va .ng on po 6.4 Identification of Campylobacter anonvex with a and co d nding en usually 1 to 2 mm in diameter but may be pinpoint to sey veral mm in 48 hete acte bic annot be picked immediately. Use a platinum or plastic needle to pick three suspect Campylobacter 10 ml of brucella-FBP () th can var greatly in colonial morphology,it is advisable to similarly culture at least one or all colony types present on the plates to assure the target no overlo ening isolates. by loosened for 24 to 48hat42±1.0 c in an atmosphere of5号o2 introduce oxygon Do not mediare tubes of campylobacte La Perform the following identification tests on each BrBP broth culture: a. the BFBP 1 immersion obiective. Young cells of Campylobacter appear long). omn8uorganod8o2panine2h1tan9e as narrow curved rods (0.2 to 0.8 corkscrew-like motility. Pairs of ce 11s can resemble the of a9u11 span or che ette fown for more than 72 h my become non-culturabieand Socsoid. Campylobacters are Gram negative,but Gram staining may 6-5
USDA/FSIS Microbiology Laboratory Guidebook 3rd Edition/1998 6-5 ii. CampyPak PlusÔ (BBL) or Gas Generating Kits for Campylobacter (Oxoid). Follow the manufacturer's instructions on use and disposal of the kit materials. Keep jars away from flames when opening. NOTE: Gas generator envelopes should be used if non-vented anaerobic jars are the only type available. Evacuation-replacement gassing of vented anaerobic jars is very economical. To facilitate lid removal from a vented anaerobic jar, first release pressure by opening clamped tubing on port or by depressing the valve stem. 6.4 Identification of Campylobacter Campylobacter colonies on MCCDA are smooth, shiny, and convex with a defined edge, or flat, transparent or translucent, and spreading with an irregular edge; colorless to grayish or light cream; and usually 1 to 2 mm in diameter but may be pinpoint to several mm in diameter. Plates of Campylobacter colonies may be stored up to 48 h refrigerated under microaerobic conditions if isolates cannot be picked immediately. Use a platinum or plastic needle to pick three suspect Campylobacter colonies for each sample from the MCCDA plates and transfer each to 10 ml of brucella-FBP (BFBP) broth. Since campylobacters can vary greatly in colonial morphology, it is advisable to similarly culture at least one or all colony types present on the plates to assure the target is not overlooked. Alternatively, direct screening of colonies by phase-contrast microscopy can be done prior to picking isolates. To culture isolates, incubate the BFBP tubes with caps loosened for 24 to 48 h at 42 ± 1.0o C in an atmosphere of 5% O2, 10% CO2, and 85% N2. Do not vortex culture tubes of Campylobacter, this will introduce oxygen into the media. Perform the following identification tests on each BFBP broth culture: a. Examine a wet-mount preparation of the BFBP broth culture with a phase-contrast microscope using a 100X oil immersion objective. Young cells of Campylobacter appear as narrow curved rods (0.2 to 0.8 mm wide by 1.5 to 5 mm long). The organisms show rapid movement with darting or corkscrew-like motility. Pairs of cells can resemble the silhouette of a gull's wing span or the letter S. Longer chains can appear helically curved, and multispiralled filamentous elongated forms may exist. Cells grown for more than 72 h may become non-culturable and coccoid. Campylobacters are Gram negative, but Gram staining may

USDA/FSIS Microbiology Laboratory Guidebook 3rd Edition/1998 be omitted since celi morphology and motility are more (carboi fuchsin [used instead of safranin as a counter stain to improve Gram stain results.) Continue nap cuiturea that axhibit typical b. Inoculate the top 10 mm laver of a tube of semisolid brucella glucose medium with several drops of the above BFBP broth culture. Inc ubate tur es with r microaerob: i. Glucose fermentation test: are ative,so t w11 acid with ed indicator)in the semisolid brucella glucose medium. ii. Catalase test: After readi ing the resul of the peroxide to the emisolid brucella glucose culture,let sit for two to three minutes, then gently invert the tube to distribute the rea gent 6 C. and C.coli are catalase positive. Add ut six drops of che BFBP broth a sterile swab a bent glass rod. Ase ptically place disc of nalidixic acid (30 and a dis of ce halothin 30ug) μg) each P1at ss ch disc with plates in an naerobic aat72±08orba5d in a microaerobic atmosphere. i. Susceptibility to nalidixic acid and antibiotic impr C.jejuni and C.coli are sensitive to nalidixic acid and I a clear zone of inhibition n11 exist aro the disc. A zone of to sen present right up disc. gro th will to the Lawns of acter growth may be very light and can be to tilt the plate ight for vi ii.Oxidase test:place a 2 cm square piece of filter 6-6
USDA/FSIS Microbiology Laboratory Guidebook 3rd Edition/1998 6-6 be omitted since cell morphology and motility are more significant in the identification of these organisms. (Carbol fuchsin [0.5%] is used instead of safranin as a counter stain to improve Gram stain results.) Continue confirmation of those BFBP cultures that exhibit typical Campylobacter morphology. b. Inoculate the top 10 mm layer of a tube of semisolid brucella glucose medium with several drops of the above BFBP broth culture. Incubate tubes with caps loosened in an anaerobic jar under microaerobic conditions at 42 ± 1.0o C for 1 to 3 days. i. Glucose fermentation test: Campylobacters are nonfermentative, so the color of the medium will remain red-orange. A positive reaction shows a yellow color (acid with phenol red indicator) in the semisolid brucella glucose medium. ii. Catalase test: After reading the results of the glucose fermentation test, add 1 ml of 3% hydrogen peroxide to the semisolid brucella glucose medium culture, let sit for two to three minutes, then gently invert the tube to distribute the reagent. Examine after 1 to 10 minutes for formation of bubbles, indicating a positive reaction. C. jejuni and C. coli are catalase positive. c. Add about six drops of the BFBP broth culture to a BFBP agar plate, and spread the inoculum over the surface with a sterile swab or a bent glass rod. Aseptically place a disc of nalidixic acid (30 mg) and a disc of cephalothin (30 mg) on each plate. Press each disc with sterile forceps to adhere it to the agar surface. Incubate the plates in an anaerobic jar at 42 ± 1.0o C for 1 to 3 days in a microaerobic atmosphere. i. Susceptibility to nalidixic acid and cephalothin: Observe the growth patterns surrounding the antibiotic impregnated discs. C. jejuni and C. coli are sensitive to nalidixic acid, and a clear zone of inhibition will exist around the disc. A zone of any size indicates sensitivity. The organisms are both resistant to cephalothin, so growth will be present right up to the disc. Lawns of Campylobacter growth may be very light and can be difficult to see, so it is helpful to tilt the plate at an angle under a light for viewing. ii. Oxidase test: Place a 2 cm square piece of filter

USDA/FSIS Microbiology Laboratory Guidebook 3rd Edition/1998 from the e。etr ih and doo5 Heavily above agar plate onto the ssagent-impregnated paper in to 5 mm in ng a pl or p. The test ce Alternatively the PD1co test may be used. Campylobacters d. Optional tests bioche 21 seful for pylobacters include nitrite re H2S production, in growth NaCl an 10m 2 9 ith darting motility Biochemically, they are catalase positive, oxidase acid sensitive,anc ng bet since both are causes of human campylobacteriosis. tests to separate Hippu: appea se t apid nethod available (Cacho et al. 1989) jejuni is positive for this test,while C.coli yields a negative reaction. 6-7
USDA/FSIS Microbiology Laboratory Guidebook 3rd Edition/1998 6-7 paper in an empty petri dish and add 1 to 2 drops of oxidase reagent to the paper. Heavily smear cells from the above BFBP agar plate onto the reagent-impregnated paper in a spot 3 to 5 mm in diameter using a platinum or plastic loop. The test is positive if the cell mass turns dark purple within 30 seconds. Alternatively, the Difco DrySlideÔ oxidase test may be used. Campylobacters are oxidase positive. d. Optional tests Other biochemical tests useful for differentiation of catalase-positive campylobacters include nitrate and nitrite reduction, H2S production, growth in 1% glycine, growth in 3.5% NaCl, and growth at 25, 30.5, 37, and 42o C. C. jejuni/coli grow well at 42o C and are curved or S-shaped with darting, corkscrew-like motility. Biochemically, they are catalase positive, oxidase positive, nonfermentative, nalidixic acid sensitive, and cephalothin resistant. Distinguishing between C. jejuni and C. coli is usually not necessary in a food microbiology laboratory since both are causes of human campylobacteriosis. The few existing tests to separate these species are not dependable. Hippurate hydrolysis appears to be the most reliable and useful test for this purpose. A convenient rapid disk method is available (Cacho et al., 1989). C. jejuni is positive for this test, while C. coli yields a negative reaction
USDA/FSIS Microbiology Laboratory Guidebook 3rd Edition/1998 6.5 Multiple Start Days a Monday Saturday;starting either day will the table below according to the day Analysis To Be Done On Days Storting Enrichment Plating Coone ohc Read/ Tests MON MON TUE WED THU FRI TUE TUE WED THU FRI MON WED WED THU FRI MON WED THU THU FRI MON TUE THU FRI FRI SAT MON TUE THU SAT SAT SUN MoN TUE THU 6.6 Storage and Transport of Stock Cultures overnight BFBP broth cultures into 42±102 in an atmos f"562.10%c02 refrigerated under this atmosphere for up to a month without serial passage. Cultures in this medium can be transported by mail. Seal caps with adhesive tape to prevent leakage during Cultures grown in enriched semisolid brucella medium may be stored under atmospheric conditions at room is also guitabis with Cultures may aiso be preserved frozen. To prepare these stocks 6 drops of a agar plate an the。1at and' nd Brucella broth with 158 sterile glycerol. The suspension can be months stocks w 6-8
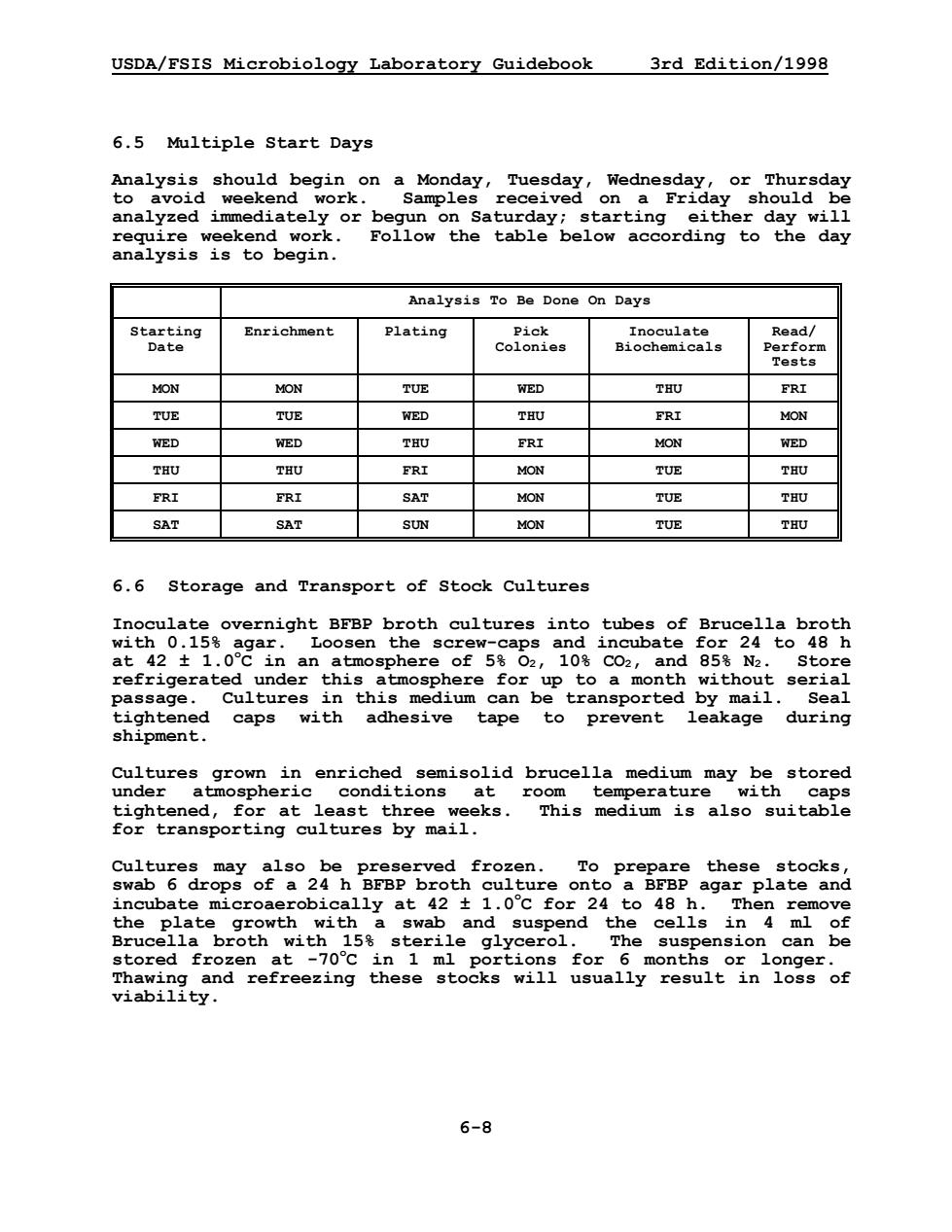
USDA/FSIS Microbiology Laboratory Guidebook 3rd Edition/1998 6-8 6.5 Multiple Start Days Analysis should begin on a Monday, Tuesday, Wednesday, or Thursday to avoid weekend work. Samples received on a Friday should be analyzed immediately or begun on Saturday; starting either day will require weekend work. Follow the table below according to the day analysis is to begin. Analysis To Be Done On Days Starting Date Enrichment Plating Pick Colonies Inoculate Biochemicals Read/ Perform Tests MON MON TUE WED THU FRI TUE TUE WED THU FRI MON WED WED THU FRI MON WED THU THU FRI MON TUE THU FRI FRI SAT MON TUE THU SAT SAT SUN MON TUE THU 6.6 Storage and Transport of Stock Cultures Inoculate overnight BFBP broth cultures into tubes of Brucella broth with 0.15% agar. Loosen the screw-caps and incubate for 24 to 48 h at 42 ± 1.0o C in an atmosphere of 5% O2, 10% CO2, and 85% N2. Store refrigerated under this atmosphere for up to a month without serial passage. Cultures in this medium can be transported by mail. Seal tightened caps with adhesive tape to prevent leakage during shipment. Cultures grown in enriched semisolid brucella medium may be stored under atmospheric conditions at room temperature with caps tightened, for at least three weeks. This medium is also suitable for transporting cultures by mail. Cultures may also be preserved frozen. To prepare these stocks, swab 6 drops of a 24 h BFBP broth culture onto a BFBP agar plate and incubate microaerobically at 42 ± 1.0o C for 24 to 48 h. Then remove the plate growth with a swab and suspend the cells in 4 ml of Brucella broth with 15% sterile glycerol. The suspension can be stored frozen at -70o C in 1 ml portions for 6 months or longer. Thawing and refreezing these stocks will usually result in loss of viability