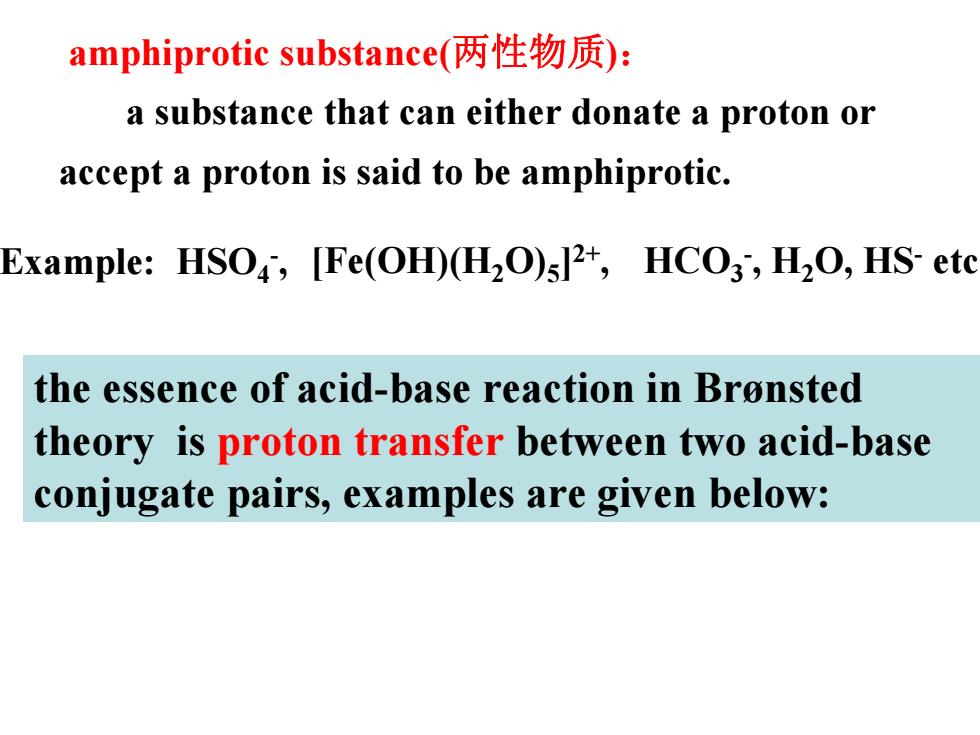
amphiprotic substance(两性物质): a substance that can either donate a proton or accept a proton is said to be amphiprotic. Example:HSO,[Fe(OH)(H2O)512,HCO3,H2O,HS-etc the essence of acid-base reaction in Bronsted theory is proton transfer between two acid-base conjugate pairs,examples are given below:
amphiprotic substance(两性物质 ) : a substance that can either donate a proton or accept a proton is said to be amphiprotic. HCO 3 -, H 2O, HS - [Fe(OH)(H etc 2O) 5 ]2+ HSO , 4 - Example: , the essence of acid-base reaction in Brønsted theory is proton transfer between two acid-base conjugate pairs, examples are given below:
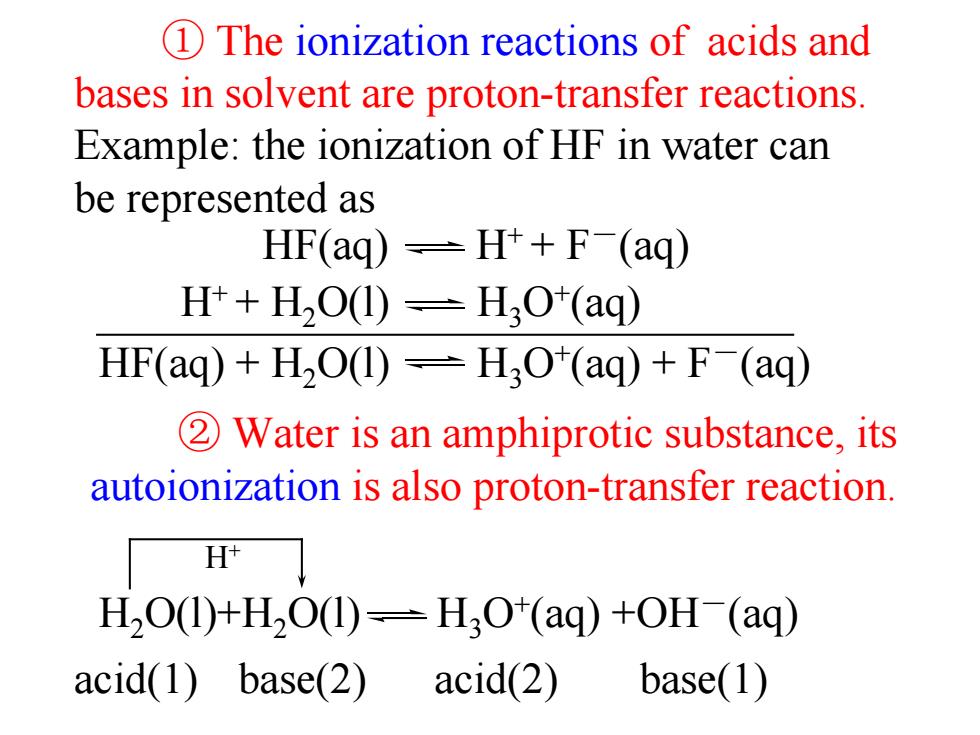
1 The ionization reactions of acids and bases in solvent are proton-transfer reactions. Example:the ionization of HF in water can be represented as HF(ag)-H++F(aq) H++H2O(1)-H;O*(aq) HF(aq)+H2O(1)=H3O*(aq)+F(aq) 2Water is an amphiprotic substance,its autoionization is also proton-transfer reaction. H H2O(1)+H2O(1)=H3O*(aq)+OH-(aq) acid(1)base(2) acid(2) base(1)
① The ionization reactions of acids and bases in solvent are proton-transfer reactions. Example: the ionization of HF in water can be represented as ② Water is an amphiprotic substance, its autoionization is also proton-transfer reaction. H+ acid(1) base(2) acid(2) base(1) HF(aq) H+ + F-(aq) H+ + H2O(l) H3O+(aq) HF(aq) + H2O(l) H3O+(aq) + F-(aq) H2O(l)+H2O(l) H3O+(aq) +OH-(aq)
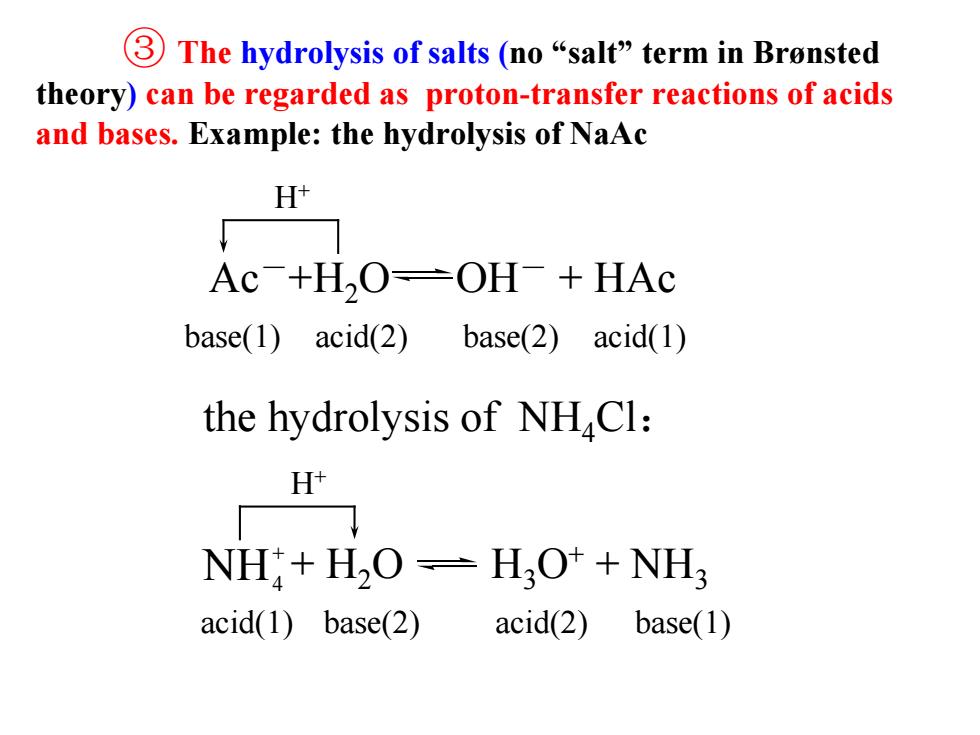
③The hydrolysis of salts(no“salt”term in Bronsted theory)can be regarded as proton-transfer reactions of acids and bases.Example:the hydrolysis of NaAc H Ac+H,O-OH+HAc base(1)acid(2)base(2)acid(1) the hydrolysis of NHCl: H NH+H2O=H2O++NH acid(1)base(2) acid(2) base(1)
③ The hydrolysis of salts (no “salt” term in Brønsted theory) can be regarded as proton-transfer reactions of acids and bases. Example: the hydrolysis of NaAc base(1) acid(2) base(2) acid(1) H+ the hydrolysis of NH4Cl: acid(1) base(2) acid(2) base(1) H+ Ac-+H2O OH- + HAc + H2O H3O+ + NH3 + NH4
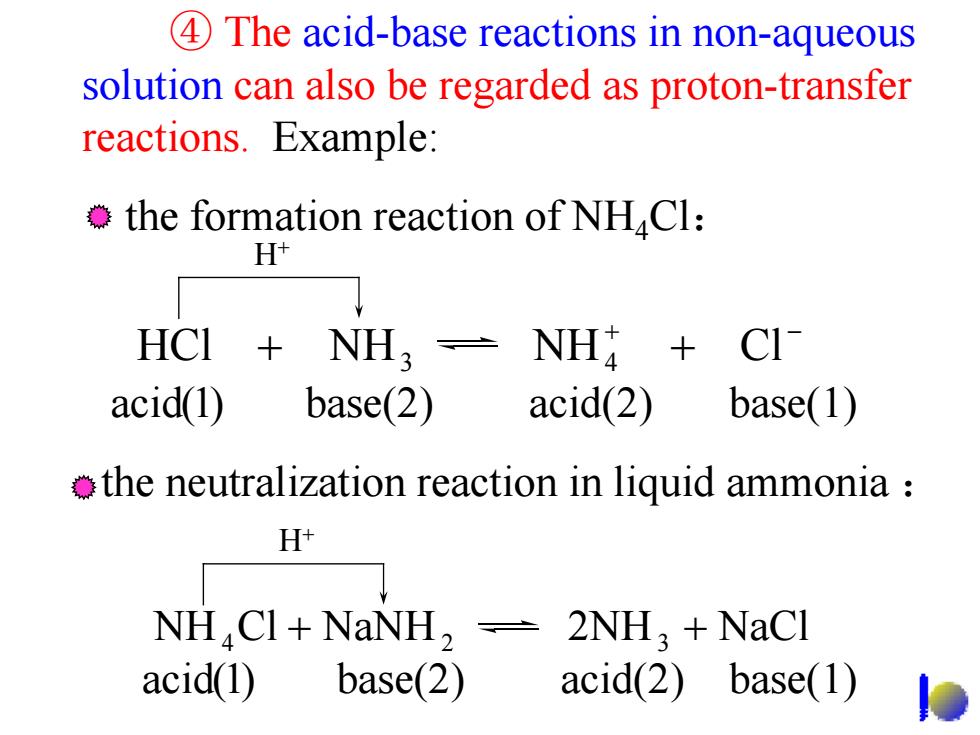
4 The acid-base reactions in non-aqueous solution can also be regarded as proton-transfer reactions.Example: the formation reaction of NHCl: H+ HCI NH NH CI acid(1) base(2) acid(2) base(1) the neutralization reaction in liquid ammonia H+ NH CI+NaNH,2NH+NaCl acid(1) base(2) acid(2) base(1)
④ The acid-base reactions in non-aqueous solution can also be regarded as proton-transfer reactions. Example: the formation reaction of NH4Cl: H+ the neutralization reaction in liquid ammonia : H+ + − HCl + NH NH + Cl 3 4 NH Cl NaNH 2NH NaCl 4 + 2 3 + acid(1) base(2) acid(2) base(1) acid(1) base(2) acid(2) base(1)
5.1.2 Relative strengths of acids and bases The strength of an acid or a base refers to the ability of donating proton for an acid or accepting proton for a base. HAc HCN HCI,HNO3 K 1.8×105 5.8×10-10 H-C1: differentiating effect((区分效应): e.g.:H2O can differentiate the relative strength of HAc,HCN. leveling effect(拉平效应):The term leveling effect refers to a solvent's ability to level the effect of a strong acid or base dissolved in it
HAc HCN differentiating effect(区分效应): e.g.:H2O can differentiate the relative strength of HAc,HCN. leveling effect(拉平效应):The term leveling effect refers to a solvent's ability to level the effect of a strong acid or base dissolved in it. The strength of an acid or a base refers to the ability of donating proton for an acid or accepting proton for a base. 5.1.2 Relative strengths of acids and bases 1.8×10 K -5 a 5.8×10-10 HCl, HNO3