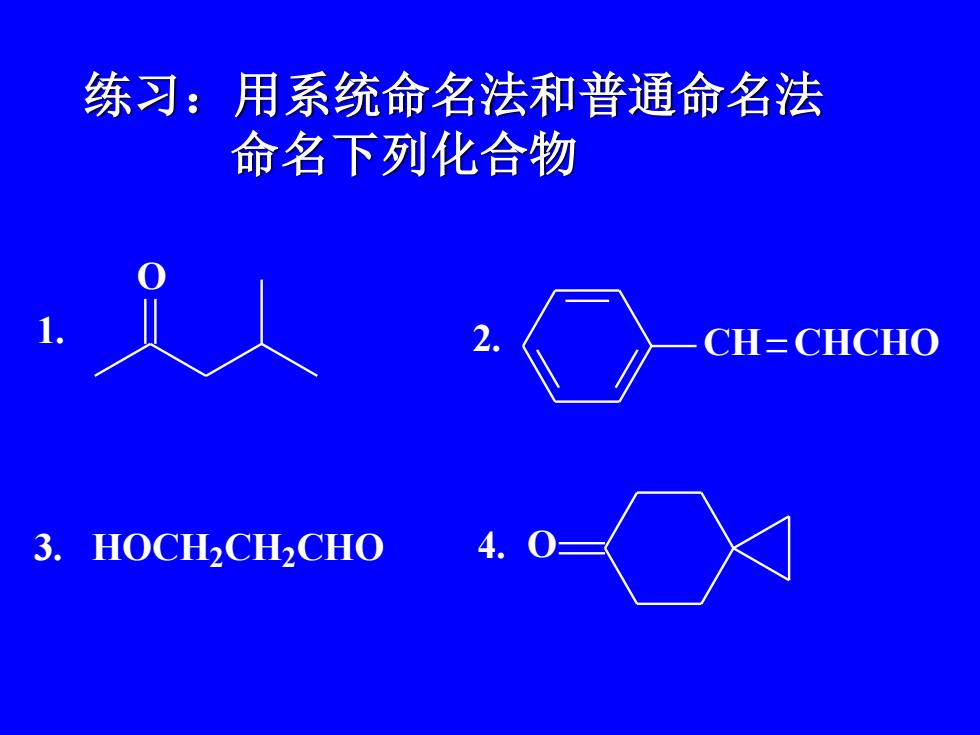
练习:用系统命名法和普通命名法命名下列化合物CH= CHCHOHOCH2CH2CHO3
练习:用系统命名法和普通命名法 命名下列化合物 1. O 2. CH CHCHO 3. HOCH2 CH2 CHO 4. O
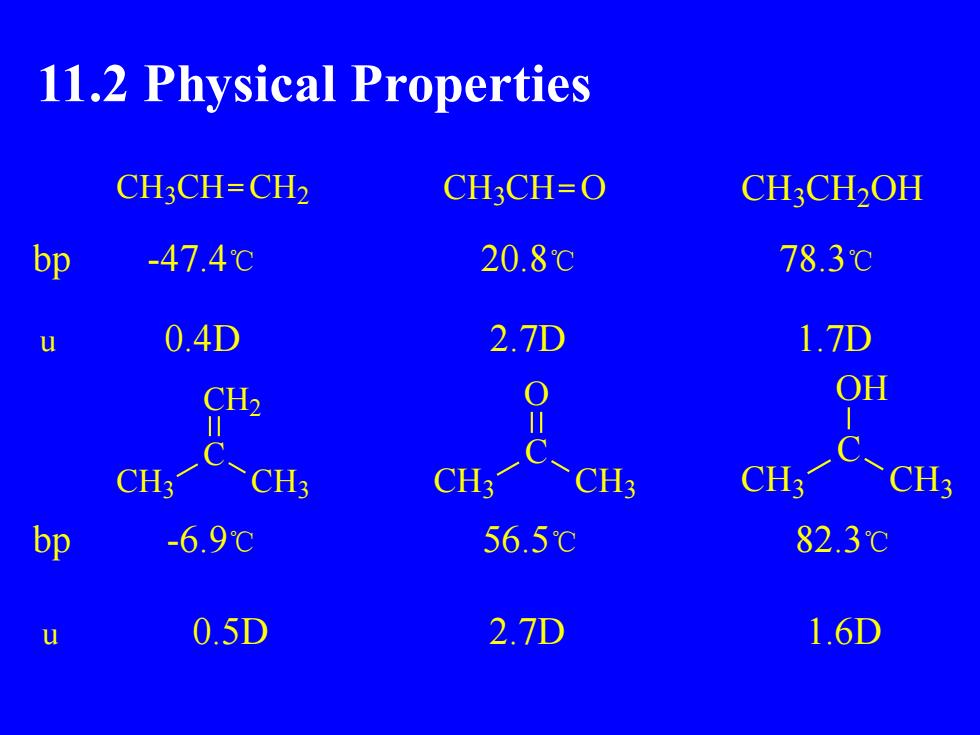
11.2 Physical PropertiesCH3CH=CH2CH3CH=OCH,CH2OH20.8℃78.3℃bp-47.4℃0.4D2.7D1.7DuOHCH2ⅡICCH3CH3CH3CH3CH3CH3-6.9℃82.3℃bp56.5℃0.5D2.7D1.6Du
11.2 Physical Properties CH3CH CH2 CH3CH O CH3CH2OH bp -47.4℃ 20.8℃ 78.3℃ u 0.4D 2.7D 1.7D C O CH3 CH3 C CH2 CH3 CH3 C OH CH3 CH3 bp -6.9℃ 56.5℃ 82.3℃ u 0.5D 2.7D 1.6D
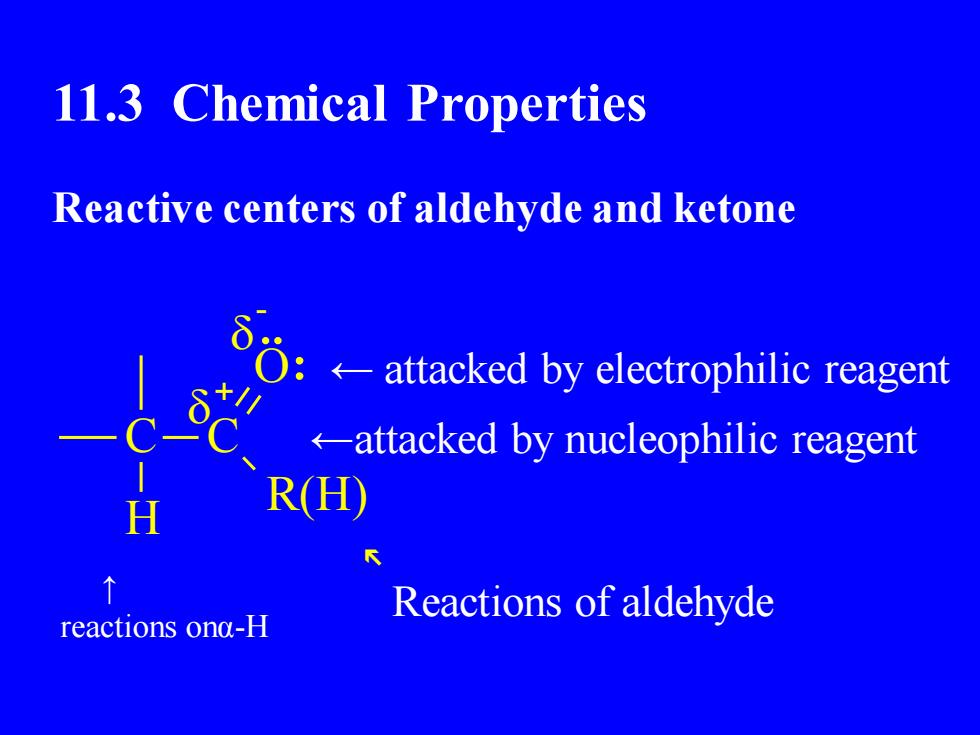
11.3 Chemical PropertiesReactive centers of aldehyde and ketoneQ:← attacked by electrophilic reagent-attackedbynucleophilicreagentR(H)HReactions of aldehydereactions onaα-H
11.3 Chemical Properties Reactive centers of aldehyde and ketone C C H R(H) O - ↑ reactions onα-H ← attacked by electrophilic reagent ←attacked by nucleophilic reagent ↖ Reactions of aldehyde
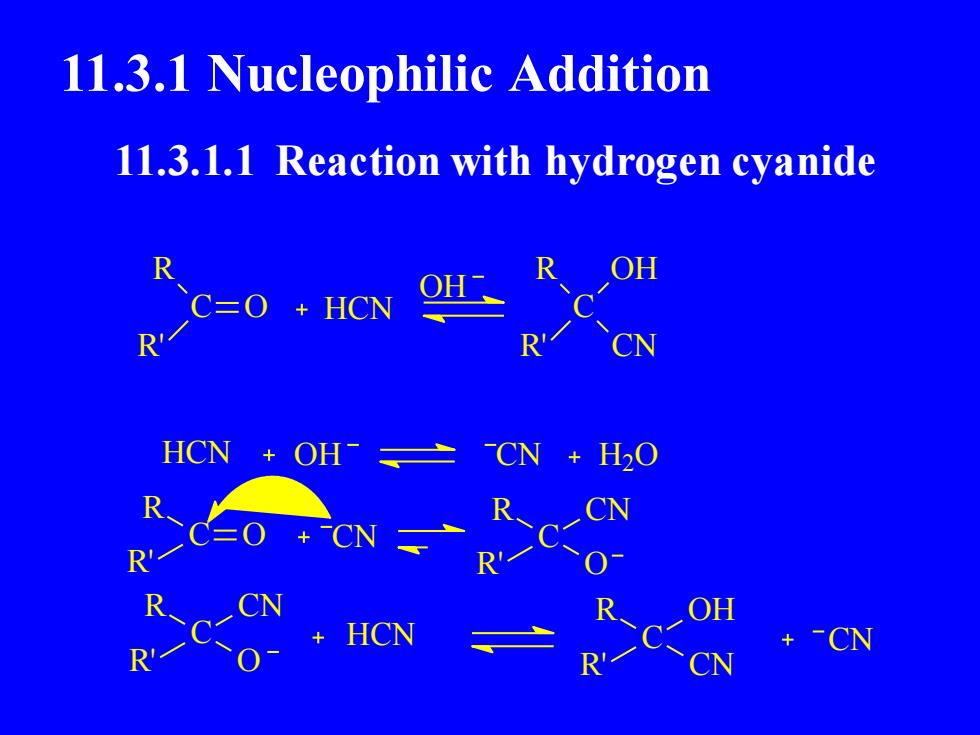
11.3.1 Nucleophilic Addition11.3.1.1 Reaction with hydrogen cyanideOHRROHC=O + HCNRRHCNOHCN+ H20RRCNCNPR0ROHHCN-CN+RRN
11.3.1 Nucleophilic Addition C O R R' HCN C R R' OH CN HCN CN H2 O C R R' O CN OH OH C R R' CN O C R R' CN O HCN C OH R' CN R CN 11.3.1.1 Reaction with hydrogen cyanide
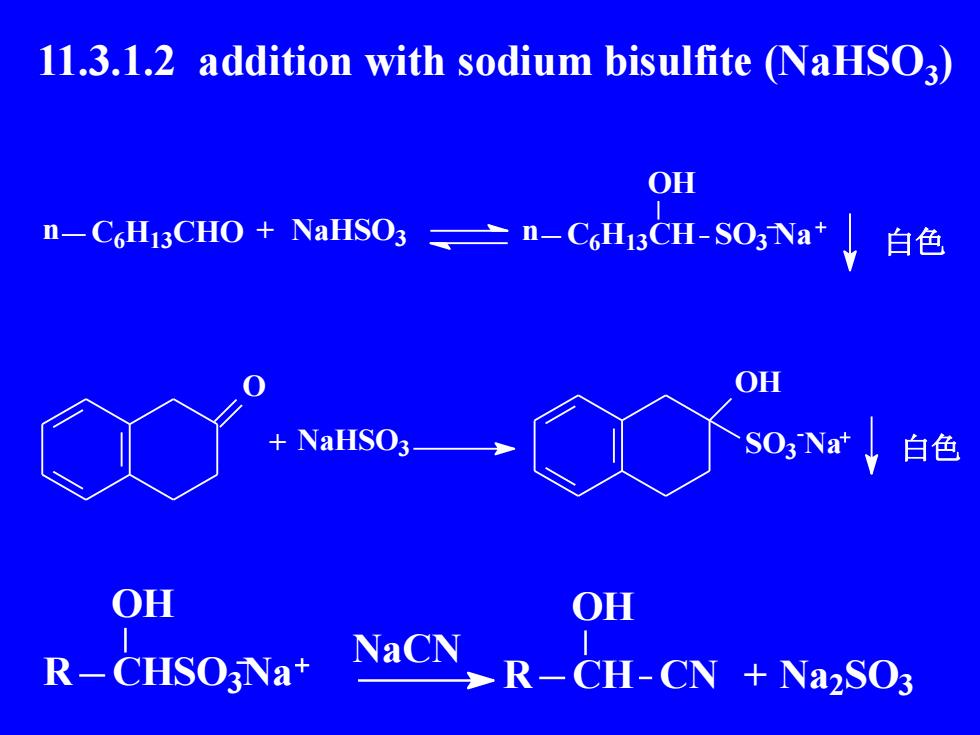
11.3.1.2 addition with sodium bisulfite (NaHSO3)OHn-CHi3CHO + NaHSO3二n-CH3CH-SO3Na白色OH+NaHSO3SO3Nat白色OHOHNaCNR-CHSO:Na+R-CH-CN + Na2SO3
11.3.1.2 addition with sodium bisulfite (NaHSO3 ) n C6H13CHO NaHSO3 n C6H13CH OH + SO3 Na 白色 O NaHSO3 OH SO3 - + Na 白色 CHSO3 Na OH R NaCN CH OH R CN + Na2 SO3