
求出切线的斜率,即微商值,可继续进行后续的数据处理 (3)坐标变换方法 在所有的图形中以直线最为简单,若两个变数间(自变数与因变数间)成直线关系, 那么此两变数的关系可以用y=ax十b来表示,x为自变数,y为因变数,a及b为常数。 常数a及b确定后即可以求出x与y的关系。如果两个变数并非直线关系,亦常常可以通 过改换变量而成直线。 例如在物理化学中常常碰到指数函数仁Axp(一,则其自然对数的表达式为: 1nk=- 衍+1n 即转换为线性方程(y=ax十b),若以1k为纵坐标,以1/T为横坐标,也可以得到一直 线。 又例如理想气体方程P=RT,经过变换绘制(P-1/)图可得直线关系 (4)图解法的应用 1)求内插值。 根据实验所得数据,以自变量作横轴,因变量作级轴,画出两变量间的关系曲线,可 在曲线所示范围内,找出与某一变量相应的另一变量的数值。如工作曲线的应用。 2)求外推值 在极限条件下,不能或不易由实验直接测得的一些物理量数值,则可利用测量数据间 的线性关系,外推至测量范围以外,求得某一函数的极限值,这种方法称为外推法。 例如:强电解质无限稀溶液的摩尔电导数值不能由实验直接测定,但可通过直接测定 多个浓度较稀溶液的摩尔电导,应用强电解质溶液理论处理实验数据,然后作图外推至浓 度为0,即可得到无限稀 等液的摩尔电导 3)作切线求函数的微商。 从曲线的斜率求函数的微商,在物化实验数据处理中经常应用。 例如:测定不同浓度溶液的表面张力后,则须从表面张力-浓度曲线上作切线,求得 定浓度时表面张力随浓度的变化率/c,然后通过吉布斯公式,计算吸附量。 4)求经验公式中的常数。 若函数间有线性关系或经函数变换后具有线性关系,均可用作图法求出式中的常数。 例如化学动力学中的阿累尼乌斯公式:素=A:exp(-E/RT) 两边取对数令其直线化(1nk=1nk列R刀,以1nk对1/T作图.可得一条直线。由直线 的斜率和截距,可分别求出活化能E和碰撞频率A的数值。 5)求函数的极值或转 「点 这是作图法的最大优点之一,物化实验数据处理中求函数的极值或转折点,均采用作 图法。 例如,二元恒沸混合物的最低或最高恒沸点及其组成的测定:大分子电解质溶液的等 电点等 4.数学方程法 一些实验数据用列表法或图形法表示后,有时还需用数学方程将实验中各变量数据间 的相互关系表示出来,这种方法称为数学方程法。 其优点在于表达方式简单,也便于求微分、积分和内插值。许多实验方程式中系数的
求出切线的斜率,即微商值,可继续进行后续的数据处理。 (3)坐标变换方法 在所有的图形中以直线最为简单,若两个变数间(自变数与因变数间)成直线关系, 那么此两变数的关系可以用y=ax+b来表示,x为自变数,y为因变数,a及b为常数。 常数a及b确定后即可以求出x与y的关系。如果两个变数并非直线关系,亦常常可以通 过改换变量而成直线。 例如在物理化学中常常碰到指数函数 k=A . exp(-E/RT),则其自然对数的表达式为: ln k= - RT E +lnA 即转换为线性方程(y=ax+b),若以 ln k 为纵坐标,以 1/T 为横坐标,也可以得到一直 线。 又例如理想气体方程 PV=nRT,经过变换绘制(P~1/V)图可得直线关系。 (4) 图解法的应用 1)求内插值。 根据实验所得数据,以自变量作横轴,因变量作纵轴,画出两变量间的关系曲线,可 在曲线所示范围内,找出与某一变量相应的另一变量的数值。如工作曲线的应用。 2)求外推值。 在极限条件下,不能或不易由实验直接测得的一些物理量数值,则可利用测量数据间 的线性关系,外推至测量范围以外,求得某一函数的极限值,这种方法称为外推法。 例如:强电解质无限稀溶液的摩尔电导数值不能由实验直接测定,但可通过直接测定 多个浓度较稀溶液的摩尔电导,应用强电解质溶液理论处理实验数据,然后作图外推至浓 度为0,即可得到无限稀溶液的摩尔电导。 3)作切线求函数的微商。 从曲线的斜率求函数的微商,在物化实验数据处理中经常应用。 例如:测定不同浓度溶液的表面张力后,则须从表面张力-浓度曲线上作切线,求得一 定浓度时表面张力随浓度的变化率 c ,然后通过吉布斯公式,计算吸附量。 4)求经验公式中的常数。 若函数间有线性关系或经函数变换后具有线性关系,均可用作图法求出式中的常数。 例如化学动力学中的阿累尼乌斯公式:k=A∙exp(-E/RT) 两边取对数令其直线化(lnk=lnA-E/RT),以 lnk 对 1/T 作图,可得一条直线。由直线 的斜率和截距,可分别求出活化能E和碰撞频率A的数值。 5)求函数的极值或转折点。 这是作图法的最大优点之一,物化实验数据处理中求函数的极值或转折点,均采用作 图法。 例如,二元恒沸混合物的最低或最高恒沸点及其组成的测定;大分子电解质溶液的等 电点等。 4. 数学方程法 一些实验数据用列表法或图形法表示后,有时还需用数学方程将实验中各变量数据间 的相互关系表示出来,这种方法称为数学方程法。 其优点在于表达方式简单,也便于求微分、积分和内插值。许多实验方程式中系数的

数值,常对应于某一物理量,因此为了求得此物理量,将数据归纳总结为经验方程式,也 是非常必要的。 ()寻求数学方程的一般方法。 当各变量间的解析依赖关系为未知时,一般可按下列步骤寻求其数学关系式: 1)确定自变量和应变量,作图,绘出曲线。 2)将所得曲线形状与已知函数的曲线形状比较。 3)根据比较结果,应用坐标变换变量方法,重新作图,使原曲线线性化 4)计算线性方程的常数。 5)若曲线无法线性化,可将原函数表示成自变量的多项式,即: =a+b+c2+d2+ 应用测量数据进行数学拟合求各常数项。多项式次数的多少,以结果能表示的可靠程度 在实验误差范围以内为准。 (②)直线方程常数的求法 1)图解法 将实验数据在直角坐标纸上作图,若得一直线,即可用线性方程式表示,其常数项可 用下述方法求得。 可在原点坐标系中将直线延长交于X轴和Y轴直接读取相应的常数项。 山。在直线上取点,应用数学的两点法求得方程的斜率和截距:为减少计算误差,所取 两点不宜相隔太近,一般在直线的两端附近选取直线上的点。 2)最小二乘法 最小二乘法的基本思路是:最佳结果应能使标准误差最小,所以残差(数学方程计算 值与测量值之差)的平方和应为最小。应用数学极值方法进行处理可得到相应的计算公式 中的常数项。最小二乘法计算虽然比较麻烦,但得到的结果可靠,应用计算机进行处理是很 易进行的。 第三节计算机的应用 计算机的使用越米越普及,相应的各种通用应用软件也很多,如Excel,Word和P 等数据和文字处理软件。很多实验的测量数据可以采用计算机处理软件进行处理并绘制简 单的直线或曲线图:也可应用专用软件进行处理数据,连接微机控制的或采集数据的实验, 如微分溶解热实验,氧弹燃烧热实验或B-2反应实验等。 详细的内容和使用请参阅专业资料和专用说明书,在此简略
数值,常对应于某一物理量,因此为了求得此物理量,将数据归纳总结为经验方程式,也 是非常必要的。 (1) 寻求数学方程的一般方法。 当各变量间的解析依赖关系为未知时,一般可按下列步骤寻求其数学关系式: 1)确定自变量和应变量,作图,绘出曲线。 2)将所得曲线形状与已知函数的曲线形状比较。 3)根据比较结果,应用坐标变换变量方法,重新作图,使原曲线线性化。 4)计算线性方程的常数。 5)若曲线无法线性化,可将原函数表示成自变量的多项式,即: y= a+bx+cx 2+dx 3+. 应用测量数据进行数学拟合求各常数项。多项式次数的多少,以结果能表示的可靠程度 在实验误差范围以内为准。 (2)直线方程常数的求法 1) 图解法 将实验数据在直角坐标纸上作图,若得一直线,即可用线性方程式表示,其常数项可 用下述方法求得。 a. 可在原点坐标系中将直线延长交于 X 轴和 Y 轴,直接读取相应的常数项。 b. 在直线上取点,应用数学的两点法求得方程的斜率和截距;为减少计算误差,所取 两点不宜相隔太近,一般在直线的两端附近选取直线上的点。 2)最小二乘法 最小二乘法的基本思路是:最佳结果应能使标准误差最小,所以残差(数学方程计算 值与测量值之差)的平方和应为最小。应用数学极值方法进行处理可得到相应的计算公式 中的常数项。最小二乘法计算虽然比较麻烦,但得到的结果可靠,应用计算机进行处理是很 易进行的。 第三节 计算机的应用 计算机的使用越来越普及,相应的各种通用应用软件也很多,如 Excel,Word 和 WPS 等数据和文字处理软件。很多实验的测量数据可以采用计算机处理软件进行处理并绘制简 单的直线或曲线图;也可应用专用软件进行处理数据,连接微机控制的或采集数据的实验, 如微分溶解热实验,氧弹燃烧热实验或 B-Z 反应实验等。 详细的内容和使用请参阅专业资料和专用说明书,在此简略

Experiment2 Saturated Vapor Pressure of Pure Liquids 1.Objective 1.the definition of vapor pressure for pureliquids and the concepto equilibrium between gas and liquid: 1.2 To gain an insight into the relationship between temperature and the saturated vapor pressure ofliquids-Clausius-Clapeyron quation 1.3 To determine the saturated vapor pressure of cyclohexane at different temperature using vacuum gauge,to master the elementary experimental technique for low vacuum operation. 1.4 To learn the way to obtain the average molar enthalpy of vaporization of the examine liquid over a range of temperatures using graphical method as well as its normal boiling point under ambient pressure. 2.Introduction The saturated vapor pressure of a pure liquid is defined as the pressure of the vapor existing in equilibrium with the liquid at a specified temperature.Here,the equilibrium state refers to a .Ata specified temperature.system inasaled vacuum vessel the molecular species escape from the liquid surface forming the vapor phase.and meanwhile the liquid is regenerated by condensation of the vapor molecules through collision.A will be attained if the rates of the above two processes becomequ and thereafter the density of the vapor in the gas phase will retain a fixed value.which is called the sauration vapor pressure of the liquid. The vapor pressure of a pure liquid varies with temperature.Their relation cou be expressed as the Clausius-Clapeyron equation dinp=ArHa (2-2-1 RT Where p is the vapor pressure of the pure liquid at temperature T.T is the thermodynamic temperature:AHis the molar enthalpy of vaporization,R is the ideal gas constant.Within a moderate range of be considered as a constant,namely,the average
Experiment 2 Saturated Vapor Pressure of Pure Liquids 1. Objective 1.1 To comprehend the definition of saturated vapor pressure for pure liquids and the concept of equilibrium between gas and liquid; 1.2 To gain an insight into the relationship between temperature and the saturated vapor pressure of liquids—Clausius-Clapeyron equation. 1.3 To determine the saturated vapor pressure of cyclohexane at different temperature using vacuum gauge; to master the elementary experimental technique for low vacuum operation. 1.4 To learn the way to obtain the average molar enthalpy of vaporization of the examine liquid over a range of temperatures using graphical method as well as its normal boiling point under ambient pressure. 2. Introduction The saturated vapor pressure of a pure liquid is defined as the pressure of the vapor existing in equilibrium with the liquid at a specified temperature. Here, the equilibrium state refers to a dynamic equilibrium. At a specified temperature, for a liquid system occluded in a sealed vacuum vessel the molecular species escape from the liquid surface forming the vapor phase, and meanwhile the liquid is regenerated by condensation of the vapor molecules through collision. A dynamic equilibrium will be attained if the rates of the above two processes become equal, and thereafter the density of the vapor in the gas phase will retain a fixed value, which is called the saturation vapor pressure of the liquid. The vapor pressure of a pure liquid varies with temperature. Their relation could be expressed as the Clausius-Clapeyron equation dT d ln p * = 2 RT V Hm (2-2−1) Where P* is the vapor pressure of the pure liquid at temperature T; T is the thermodynamic temperature; V Hm is the molar enthalpy of vaporization; R is the ideal gas constant. Within a moderate range of temperature, V Hm could be considered as a constant, namely, the average

molar enthalpy of vaporization.Integrating the Equation(2-2-1).one can obtain: np=-△2+C (2-2-2) RT where Cis the integration constant,which is related to the units of p* From Equation (2-2-2),it is obvious that after measuring the vapor pressures at various temperatures within a moderate temperature range,a straight line can be obtained by plotting against 1/T.The average molar enthalpy of vaporization ,H of the liquid in this temperature range could be estimated from the slope of the straight line.The temperature at which the saturated vapor pressure of the liquid becomes equal to ambient pressure(101.325 kPa)is called the normal boiling point of the liquid Thus,the plot of the straight line can also be used to obtain the normal boiling point of the liquid. There are a number of ways to measure vapor pressures,such as the dynamic method,the static method,the saturated flow method and etc.In this experiment the static method is used,ie,the liquid is paced in a sealed system,and then its vapor pressures at various temperatures and boiling points at various pressures are measured.This method is appropriate for measuring liquids with relatively large vapor pressures. 3.Apparatus and Reagent Apparatus for vapor pressure determination;Barometer with digital output,Thermometer(two pieces):Magnetic stirrer:Vacuum pump;Electric heater,Vacuum gauge with digital output. Cyclohexane(AR Procedure 4.1.Setting up the apparatus Make sure that all air-tight.The balance tube comprises three glass tubes,i.e tube a b and c.The liquid is stored in tubea and the two connected tubes band care
molar enthalpy of vaporization. Integrating the Equation (2-2-1), one can obtain: ln p = − R V Hm C T + 1 (2-2−2) where C is the integration constant, which is related to the units of P*. From Equation (2-2-2), it is obvious that after measuring the vapor pressures at various temperatures within a moderate temperature range, a straight line can be obtained by plotting lnP* against 1/T. The average molar enthalpy of vaporization v Hm of the liquid in this temperature range could be estimated from the slope of the straight line. The temperature at which the saturated vapor pressure of the liquid becomes equal to ambient pressure (101.325 kPa) is called the normal boiling point of the liquid. Thus, the plot of the straight line can also be used to obtain the normal boiling point of the liquid. There are a number of ways to measure vapor pressures, such as the dynamic method, the static method, the saturated flow method and etc. In this experiment the static method is used, i.e., the liquid is placed in a sealed system, and then its vapor pressures at various temperatures and boiling points at various pressures are measured. This method is appropriate for measuring liquids with relatively large vapor pressures. 3. Apparatus and Reagent Apparatus for vapor pressure determination; Barometer with digital output; Thermometer (two pieces); Magnetic stirrer; Vacuum pump; Electric heater; Vacuum gauge with digital output; Cyclohexane (AR). 4. Procedure 4.1. Setting up the apparatus Make sure that all connections are air-tight. The balance tube comprises three interconnected glass tubes, i.e. tube a, b and c. The liquid is stored in tube a, and the two connected tubes b and c are
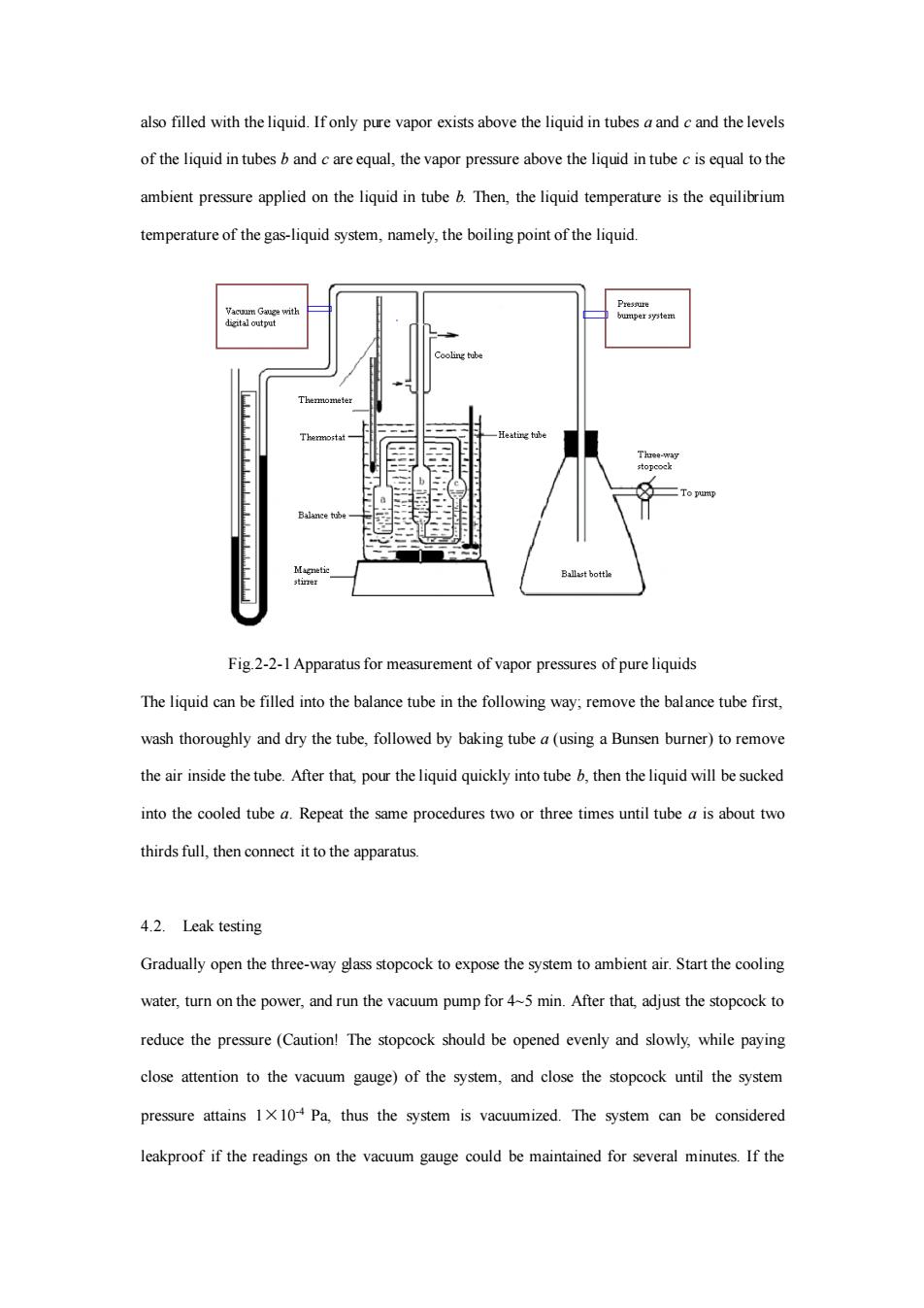
also filled with the liquid.Ifonly pure vapor exists above the liquid in tubes aand cand the levels of the liquid in tubesandareequal,the vapor pressure above the liquid in tubeisequal tothe ambient pressure applied on the liquid in tube b.Then,the liquid temperature is the equilibrium temperature of the gas-liquid system,namely,the boiling point of the liquid. Fig 2-2-1 Apparatus for measurement of vapor pressures of pure liquids The liquid can be flled into the balance tube in the following way,remove the balance tube first. wash thoroughly and dry the tube,followed by baking tube(using a Bunsen burer)to remove the air inside the tube.After that pour the liquid quickly into tube b.then the liquid will be sucked into the cooed tube.Repeat the same procedures two or three times until tube is about two thirds full,then connect it to the apparatus. 4.2.Leak testing Gradually open the three-way glass stopcock to expose the system to ambient air.Start the cooling water,turn on the power,and run the vacuum pump for 45 min.After that,adjust the stopcock to reduce the pressure(Caution!The stopcock should be opened evenly and slowly,while paying close attention to the vacuum gauge)of the system,and close the stopcock until the system pressure attains 1 Pa thus the system is vacuumized.The system can be considered leakproof if the readings on the vacuum gauge could be maintained for several minutes If the
also filled with the liquid. If only pure vapor exists above the liquid in tubes a and c and the levels of the liquid in tubes b and c are equal, the vapor pressure above the liquid in tube c is equal to the ambient pressure applied on the liquid in tube b. Then, the liquid temperature is the equilibrium temperature of the gas-liquid system, namely, the boiling point of the liquid. Fig.2-2-1 Apparatus for measurement of vapor pressures of pure liquids The liquid can be filled into the balance tube in the following way; remove the balance tube first, wash thoroughly and dry the tube, followed by baking tube a (using a Bunsen burner) to remove the air inside the tube. After that, pour the liquid quickly into tube b, then the liquid will be sucked into the cooled tube a. Repeat the same procedures two or three times until tube a is about two thirds full, then connect it to the apparatus. 4.2. Leak testing Gradually open the three-way glass stopcock to expose the system to ambient air. Start the cooling water, turn on the power, and run the vacuum pump for 4~5 min. After that, adjust the stopcock to reduce the pressure (Caution! The stopcock should be opened evenly and slowly, while paying close attention to the vacuum gauge) of the system, and close the stopcock until the system pressure attains 1×10-4 Pa, thus the system is vacuumized. The system can be considered leakproof if the readings on the vacuum gauge could be maintained for several minutes. If the