
3.1热力学术语和基本概念 3.1.7广度性质与强度性质 广度性质:与物质的量有关,具有加和性 如:V等;又称为容量性质 强度性质:与物质的量无关,不具有加和性 如:T等
3.1.7 广度性质与强度性质 广度性质:与物质的量有关,具有加和性 如:V 等;又称为容量性质 强度性质:与物质的量无关,不具有加和性 如:T 等 3.1 热力学术语和基本概念

3.1热力学术语和基本概念 3.1.8化学反应计量式和反应进度 1.反应计量式 dD卡eE=fF+gG 0=-dD-eE+fF+gG VBB 0 式中:B为参加化学反应的各物质 为物质B的化学计量系数(产物为正,原料为负) VD=-d VE=-e VE=f VG=g
𝑑D + 𝑒E = 𝑓F + 𝑔G 3.1.8 化学反应计量式和反应进度 式中:B为参加化学反应的各物质 B为物质B 的化学计量系数(产物为正,原料为负) 0 = −𝑑D−𝑒E + 𝑓F + 𝑔G 0 = B νBB νD = −𝑑 νE = −𝑒 νF = 𝑓 νG = 𝑔 3.1 热力学术语和基本概念 1. 反应计量式
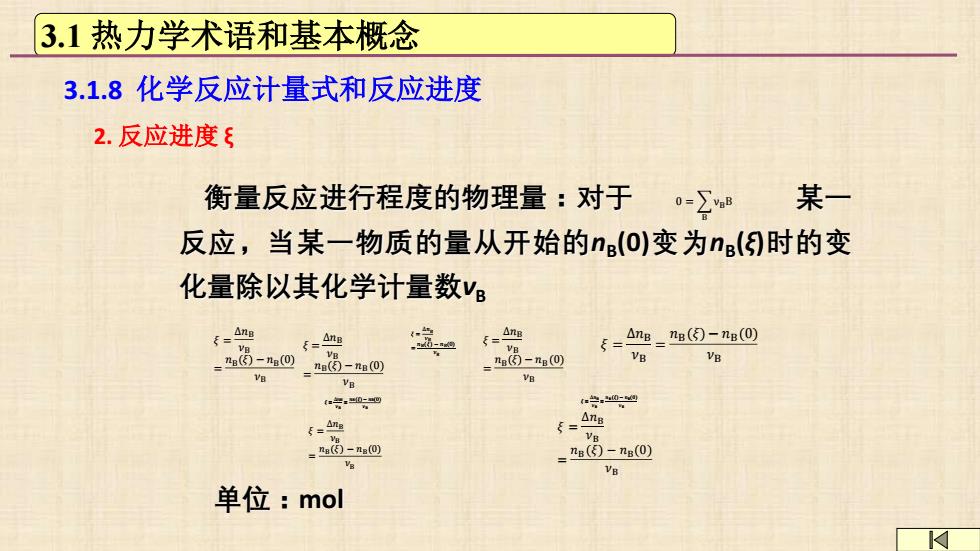
3.1热力学术语和基本概念 3.1.8化学反应计量式和反应进度 2.反应进度 衡量反应进行程度的物理量:对于 0=∑8 某一 反应,当某一物质的量从开始的n(O)变为n()时的变 化量除以其化学计量数v f、4g =Ana =Ang 5=4e=ne因-na0 -n因-0_m国-0 -t(⑤-ng(0 VB B VB VB e号0四 0四 6=Ang 4 -n的-A四 g ="n()-ng(0) ve 单位:mol
2. 反应进度 ξ 𝜉 = Δ𝑛B 𝜈B = 𝑛B(𝜉) − 𝑛B(0) 𝜈B 单位:mol 衡量反应进行程度的物理量:对于 某一 反应,当某一物质的量从开始的nB (0)变为nB (ξ)时的变 化量除以其化学计量数νB 0 = B νBB 𝜉 = Δ𝑛B 𝜈B = 𝑛B(𝜉) − 𝑛B(0) 𝜈B 𝜉 = Δ𝑛B 𝜈B = 𝑛B(𝜉) − 𝑛B(0) 𝜈B 𝜉 = Δ𝑛B 𝜈B = 𝑛B(𝜉) − 𝑛B(0) 𝜈B 𝜉 = Δ𝑛B 𝜈B = 𝑛B(𝜉) − 𝑛B(0) 𝜈B 𝜉 = Δ𝑛B 𝜈B = 𝑛B(𝜉) − 𝑛B(0) 𝜈B 𝜉 = Δ𝑛B 𝜈B = 𝑛B(𝜉) − 𝑛B(0) 𝜈B 𝜉 = Δ𝑛B 𝜈B = 𝑛B(𝜉) − 𝑛B(0) 𝜈B 𝜉 = Δ𝑛B 𝜈B = 𝑛B(𝜉) − 𝑛B(0) 𝜈B 3.1 热力学术语和基本概念 3.1.8 化学反应计量式和反应进度
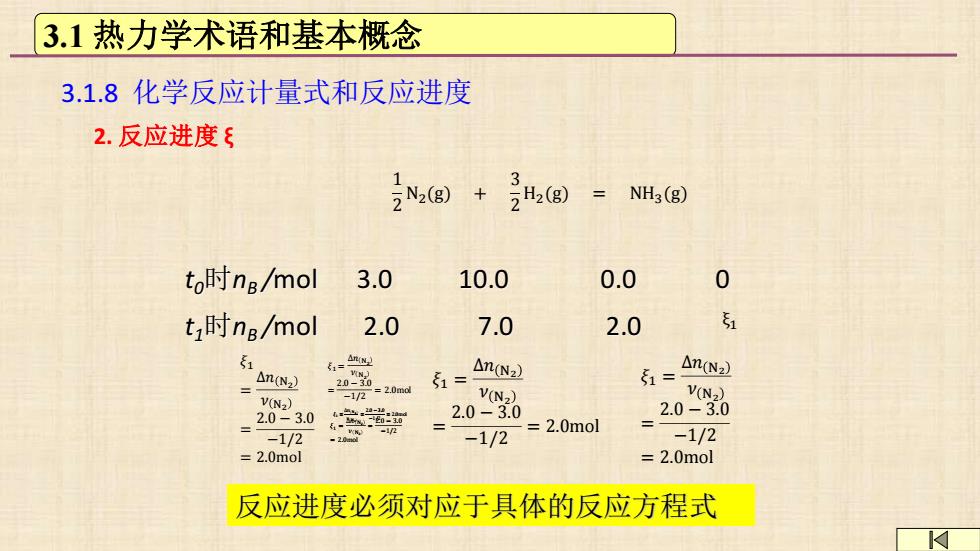
3.1热力学术语和基本概念 3.1.8化学反应计量式和反应进度 2.反应进度ξ N,g)+ 2H2(g)= NH3(g) to时ng/mol 3.0 10.0 0.0 0 t时ng/mol 2.0 7.0 2.0 51 51 名-以 n(N2 51= △n(Nz) -1/2=2.0ma 1= △n(N2 V(N2) V(N2) V(N2) 2.0-3.0 2.0-3.0 2.0-3.0 20 -2 =2.0mol -1/2 -1/2 -1/2 =2.0mol 2.0mol 反应进度必须对应于具体的反应方程式
反应进度必须对应于具体的反应方程式 𝜉1 = Δ𝑛 N2 𝜈 N2 = 2.0 − 3.0 −1/2 = 2.0mol 1 2 N2(g) + 3 2 H2(g) = NH3(g) t0时nB /mol 3.0 10.0 0.0 0 ξ1 t1时nB /mol 2.0 7.0 2.0 𝜉1 = Δ𝑛 N2 𝜈 N2 = 2.0 −3.0 −1/2 = 2.0mol 𝜉1 = Δ𝑛 N2 𝜈 N2 = 2.0 − 3.0 −1/2 = 2.0mol 𝜉1 = Δ𝑛 N2 𝜈 N2 = 2.0 − 3.0 −1/2 = 2.0mol 𝜉1 = Δ𝑛 N2 𝜈 N2 = 2.0 − 3.0 −1/2 = 2.0mol 𝜉1 = Δ𝑛 N2 𝜈 N2 = 2.0 − 3.0 −1/2 = 2.0mol 3.1 热力学术语和基本概念 3.1.8 化学反应计量式和反应进度 2. 反应进度 ξ
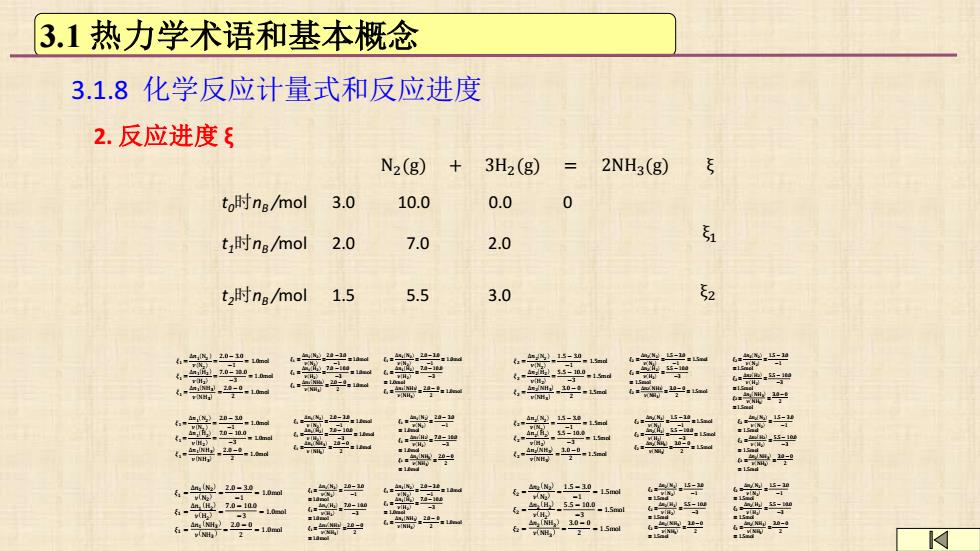
3.1热力学术语和基本概念 3.1.8化学反应计量式和反应进度 2.反应进度5 N2(g) 3H2(g) 2NH3(g) to时ng/mol 3.0 10.0 0.0 0 t时na/mol 2.0 7.0 2.0 t2时ng/mol1.5 5.5 3.0 52 tisNs28-10siaoo v(NHal a,s.0-".1ma ,.-ad 3 …. 器 学学 .ml 5-1 .2-0.10al -哥.中.sm 6 6 器学 器学
N2(g) + 3H2(g) = 2NH3(g) ξ t0时nB /mol 3.0 10.0 0.0 0 ξ1 t1时nB /mol 2.0 7.0 2.0 t2时nB /mol 1.5 5.5 3.0 ξ2 𝜉1 = Δ𝑛1 N2 𝜈 N2 = 2.0 − 3.0 −1 = 1.0mol 𝜉1 = Δ𝑛1 H2 𝜈 H2 = 7.0 − 10.0 −3 = 1.0mol 𝜉1 = Δ𝑛1 NH3 𝜈 NH3 = 2.0 − 0 2 = 1.0mol 𝜉2 = Δ𝑛2 N2 𝜈 N2 = 1.5 − 3.0 −1 = 1.5mol 𝜉2 = Δ𝑛2 H2 𝜈 H2 = 5.5 − 10.0 −3 = 1.5mol 𝜉2 = Δ𝑛2 NH3 𝜈 NH3 = 3.0 − 0 2 = 1.5mol 𝜉1 = Δ𝑛1 N2 𝜈 N2 = 2.0 − 3.0 −1 = 1.0mol 𝜉1 = Δ𝑛1 H2 𝜈 H2 = 7.0 − 10.0 −3 = 1.0mol 𝜉1 = Δ𝑛1 NH3 𝜈 NH3 = 2.0 −0 2 = 1.0mol 𝜉1 = Δ𝑛1 N2 𝜈 N2 = 2.0 − 3.0 −1 = 1.0mol 𝜉1 = Δ𝑛1 H2 𝜈 H2 = 7.0 − 10.0 −3 = 1.0mol 𝜉1 = Δ𝑛1 NH3 𝜈 NH3 = 2.0 − 0 2 = 1.0mol 𝜉2 = Δ𝑛2 N2 𝜈 N2 = 1.5 − 3.0 −1 = 1.5mol 𝜉2 = Δ𝑛2 H2 𝜈 H2 = 5.5 − 10.0 −3 = 1.5mol 𝜉2 = Δ𝑛2 NH3 𝜈 NH3 = 3.0 −0 2 = 1.5mol 𝜉2 = Δ𝑛2 N2 𝜈 N2 = 1.5 − 3.0 −1 = 1.5mol 𝜉2 = Δ𝑛2 H2 𝜈 H2 = 5.5 − 10.0 −3 = 1.5mol 𝜉2 = Δ𝑛2 NH3 𝜈 NH3 = 3.0 − 0 2 = 1.5mol 𝜉1 = Δ𝑛1 N2 𝜈 N2 = 2.0 −3.0 −1 =1.0mol 𝜉1 = Δ𝑛1 H2 𝜈 H2 = 7.0 −10.0 −3 = 1.0mol 𝜉1 = Δ𝑛1 NH3 𝜈 NH3 = 2.0 − 0 2 = 1.0mol 𝜉1 = Δ𝑛1 N2 𝜈 N2 = 2.0 −3.0 −1 = 1.0mol 𝜉1 = Δ𝑛1 H2 𝜈 H2 = 7.0 −10.0 −3 = 1.0mol 𝜉1 = Δ𝑛1 NH3 𝜈 NH3 = 2.0 − 0 2 = 1.0mol 𝜉1 = Δ𝑛1 N2 𝜈 N2 = 2.0 − 3.0 −1 = 1.0mol 𝜉1 = Δ𝑛1 H2 𝜈 H2 = 7.0 − 10.0 −3 = 1.0mol 𝜉1 = Δ𝑛1 NH3 𝜈 NH3 = 2.0 −0 2 = 1.0mol 𝜉1 = Δ𝑛1 N2 𝜈 N2 = 2.0 − 3.0 −1 = 1.0mol 𝜉1 = Δ𝑛1 H2 𝜈 H2 = 7.0 − 10.0 −3 = 1.0mol 𝜉1 = Δ𝑛1 NH3 𝜈 NH3 = 2.0 −0 2 = 1.0mol 𝜉1 = Δ𝑛1 N2 𝜈 N2 = 2.0 − 3.0 −1 =1.0mol 𝜉1 = Δ𝑛1 H2 𝜈 H2 = 7.0 − 10.0 −3 =1.0mol 𝜉1 = Δ𝑛1 NH3 𝜈 NH3 = 2.0 −0 2 =1.0mol 𝜉1 = Δ𝑛1 N2 𝜈 N2 = 2.0 −3.0 −1 = 1.0mol 𝜉1 = Δ𝑛1 H2 𝜈 H2 = 7.0 −10.0 −3 = 1.0mol 𝜉1 = Δ𝑛1 NH3 𝜈 NH3 = 2.0 − 0 2 = 1.0mol 𝜉2 = Δ𝑛2 N2 𝜈 N2 = 1.5 −3.0 −1 = 1.5mol 𝜉2 = Δ𝑛2 H2 𝜈 H2 = 5.5 −10.0 −3 = 1.5mol 𝜉2 = Δ𝑛2 NH3 𝜈 NH3 = 3.0 − 0 2 = 1.5mol 𝜉2 = Δ𝑛2 N2 𝜈 N2 = 1.5 − 3.0 −1 =1.5mol 𝜉2 = Δ𝑛2 H2 𝜈 H2 = 5.5 − 10.0 −3 =1.5mol 𝜉2 = Δ𝑛2 NH3 𝜈 NH3 = 3.0 −0 2 =1.5mol 𝜉2 = Δ𝑛2 N2 𝜈 N2 = 1.5 −3.0 −1 =1.5mol 𝜉2 = Δ𝑛2 H2 𝜈 H2 = 5.5 −10.0 −3 = 1.5mol 𝜉2 = Δ𝑛2 NH3 𝜈 NH3 = 3.0 − 0 2 = 1.5mol 𝜉2 = Δ𝑛2 N2 𝜈 N2 = 1.5 − 3.0 −1 = 1.5mol 𝜉2 = Δ𝑛2 H2 𝜈 H2 = 5.5 − 10.0 −3 = 1.5mol 𝜉2 = Δ𝑛2 NH3 𝜈 NH3 = 3.0 −0 2 = 1.5mol 𝜉2 = Δ𝑛2 N2 𝜈 N2 = 1.5 − 3.0 −1 = 1.5mol 𝜉2 = Δ𝑛2 H2 𝜈 H2 = 5.5 − 10.0 −3 = 1.5mol 𝜉2 = Δ𝑛2 NH3 𝜈 NH3 = 3.0 −0 2 = 1.5mol 𝜉2 = Δ𝑛2 N2 𝜈 N2 = 1.5 − 3.0 −1 = 1.5mol 𝜉2 = Δ𝑛2 H2 𝜈 H2 = 5.5 − 10.0 −3 = 1.5mol 𝜉2 = Δ𝑛2 NH3 𝜈 NH3 = 3.0 −0 2 = 1.5mol 3.1 热力学术语和基本概念 3.1.8 化学反应计量式和反应进度 2. 反应进度 ξ