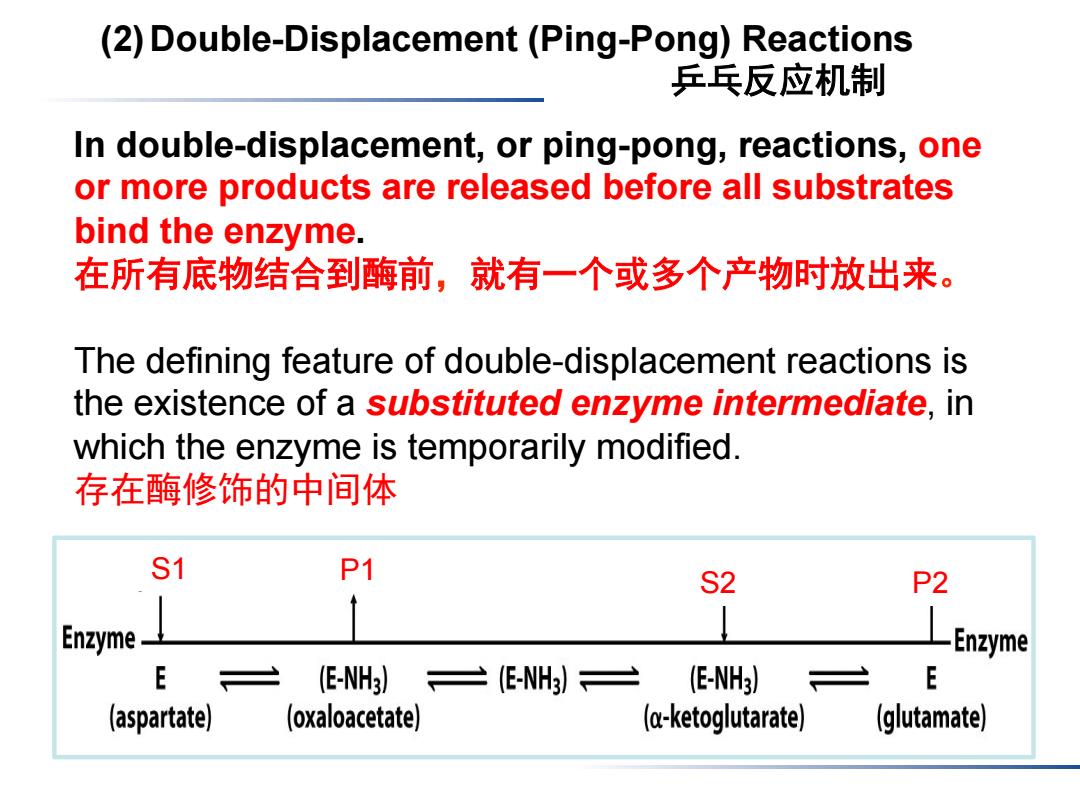
(2)Double-Displacement(Ping-Pong)Reactions 乒乓反应机制 In double-displacement,or ping-pong,reactions,one or more products are released before all substrates bind the enzyme. 在所有底物结合到酶前,就有一个或多个产物时放出来。 The defining feature of double-displacement reactions is the existence of a substituted enzyme intermediate,in which the enzyme is temporarily modified. 存在酶修饰的中间体 S1 P1 S2 P2 Enzyme Enzyme E (E-NH3) (-NH)= (E-NH3) E (aspartate) (oxaloacetate) (ketoglutarate) (glutamate)
(2)Double-Displacement (Ping-Pong) Reactions 乒乓反应机制 In double-displacement, or ping-pong, reactions, one or more products are released before all substrates bind the enzyme. 在所有底物结合到酶前,就有一个或多个产物时放出来。 The defining feature of double-displacement reactions is the existence of a substituted enzyme intermediate, in which the enzyme is temporarily modified. 存在酶修饰的中间体 S1 S2 P1 P2

Shuttle amino groups between amino acids and a-keto acids The aspartate aminotransferase门冬氨酸转氨酶 catalyzes the transfer of an amino group from aspartate to a-ketoglutarate. 00C C00- CH2 C00 CH2 H20 H29 H2 C00 —C00 H3N C00 +H3N C00 Aspartate a-Ketoglutarate Oxaloacetate Glutamate (草酰乙酸) not free enzyme Aspartate Oxaloacetate o-Ketoglutarate Glutamate Enzyme Enzyme E (E-NH3) =(-NH)三 (E-NH3) E (aspartate) (oxaloacetate) (c-ketoglutarate) (glutamate)
The aspartate aminotransferase 门冬氨酸转氨酶 catalyzes the transfer of an amino group from aspartate to α-ketoglutarate. Shuttle amino groups between amino acids and α-keto acids (草酰乙酸) not free enzyme
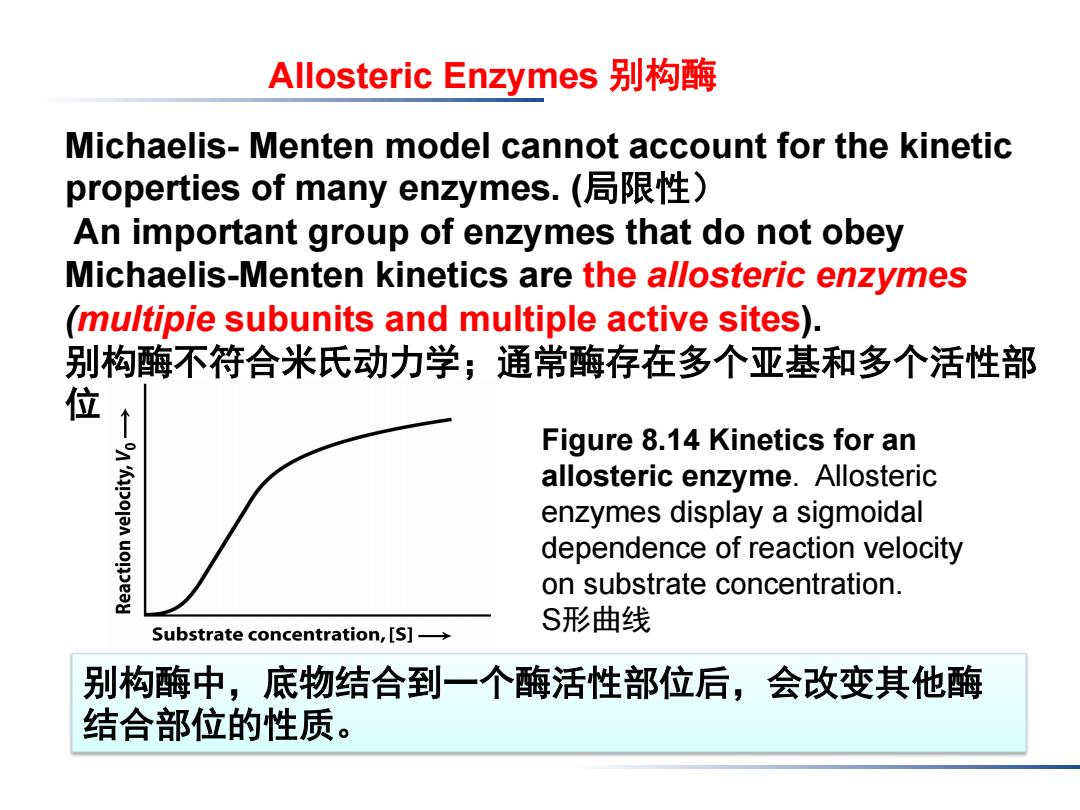
Allosteric Enzymes别构酶 Michaelis-Menten model cannot account for the kinetic properties of many enzymes.(局限性) An important group of enzymes that do not obey Michaelis-Menten kinetics are the allosteric enzymes (mu/tipie subunits and multiple active sites). 别构酶不符合米氏动力学;通常酶存在多个亚基和多个活性部 位 Figure 8.14 Kinetics for an allosteric enzyme.Allosteric enzymes display a sigmoidal dependence of reaction velocity on substrate concentration. S形曲线 Substrate concentration,[S]- 别构酶中,底物结合到一个酶活性部位后,会改变其他酶 结合部位的性质
Figure 8.14 Kinetics for an allosteric enzyme. Allosteric enzymes display a sigmoidal dependence of reaction velocity on substrate concentration. S形曲线 Allosteric Enzymes 别构酶 Michaelis- Menten model cannot account for the kinetic properties of many enzymes. (局限性) An important group of enzymes that do not obey Michaelis-Menten kinetics are the allosteric enzymes (multipie subunits and multiple active sites). 别构酶不符合米氏动力学;通常酶存在多个亚基和多个活性部 位 别构酶中,底物结合到一个酶活性部位后,会改变其他酶 结合部位的性质
Possible outcome of this interaction between subunits Cooperative effecti协同效应: the binding of substrate to one active site of the enzyme facilitates substrate binding to the other active sites. Substrate concentration,[S]- 存在别构分子: Allosteric molecule:regulatory molecules are reversibly bound to specific sites other than the catalytic sites. 别构分子:可逆结合到酶特定部位(非活性部位),起到 调节酶催化功能的作用。 酶活性可以迅速改变,因此在代谢途径中起到关键调节作 用(第10章介绍)
Cooperative effect协同效应: the binding of substrate to one active site of the enzyme facilitates substrate binding to the other active sites. Possible outcome of this interaction between subunits 存在别构分子: Allosteric molecule: regulatory molecules are reversibly bound to specific sites other than the catalytic sites. 别构分子:可逆结合到酶特定部位(非活性部位),起到 调节酶催化功能的作用。 酶活性可以迅速改变,因此在代谢途径中起到关键调节作 用(第10章介绍)

OUTLINES 1.Enzymes Are Powerful and Highly Specific Catalysts一概论 2.Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes一热力学 3.Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State一化学 4.The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes一动力学 5.Enzymes Can Be Inhibited by Specific Molecules一抑制作用
1. Enzymes Are Powerful and Highly Specific Catalysts - 概论 2. Free Energy Is a Useful Thermodynamic Function for Understanding Enzymes -热力学 3. Enzymes Accelerate Reactions by Facilitating the Formation of the Transition State -化学 4. The Michaelis-Menten Model Accounts for the Kinetic Properties of Many Enzymes -动力学 5. Enzymes Can Be Inhibited by Specific Molecules -抑制作用 OUTLINES