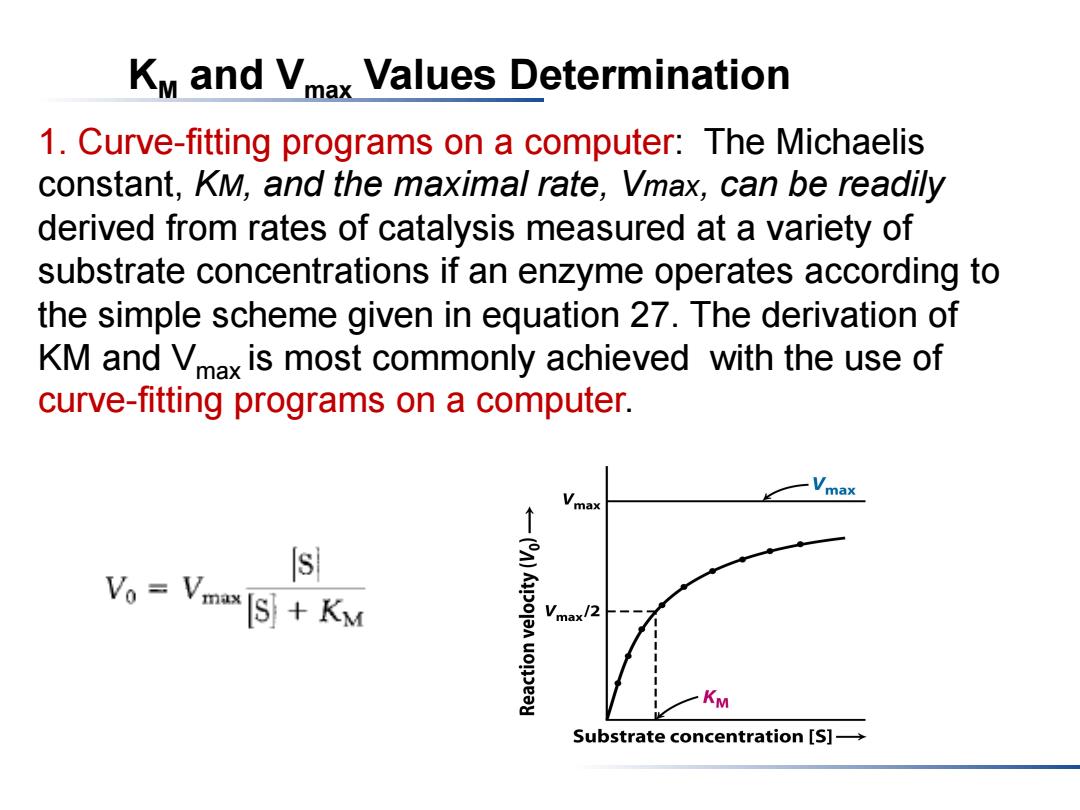
KM and Vmax Values Determination 1.Curve-fitting programs on a computer:The Michaelis constant,KM,and the maximal rate,Vmax,can be readily derived from rates of catalysis measured at a variety of substrate concentrations if an enzyme operates according to the simple scheme given in equation 27.The derivation of KM and Vmax is most commonly achieved with the use of curve-fitting programs on a computer. max s Vo Vmax [S]KM Vmax/2 KM Substrate concentration [S]-
KM and Vmax Values Determination 1. Curve-fitting programs on a computer: The Michaelis constant, KM, and the maximal rate, Vmax, can be readily derived from rates of catalysis measured at a variety of substrate concentrations if an enzyme operates according to the simple scheme given in equation 27. The derivation of KM and Vmax is most commonly achieved with the use of curve-fitting programs on a computer
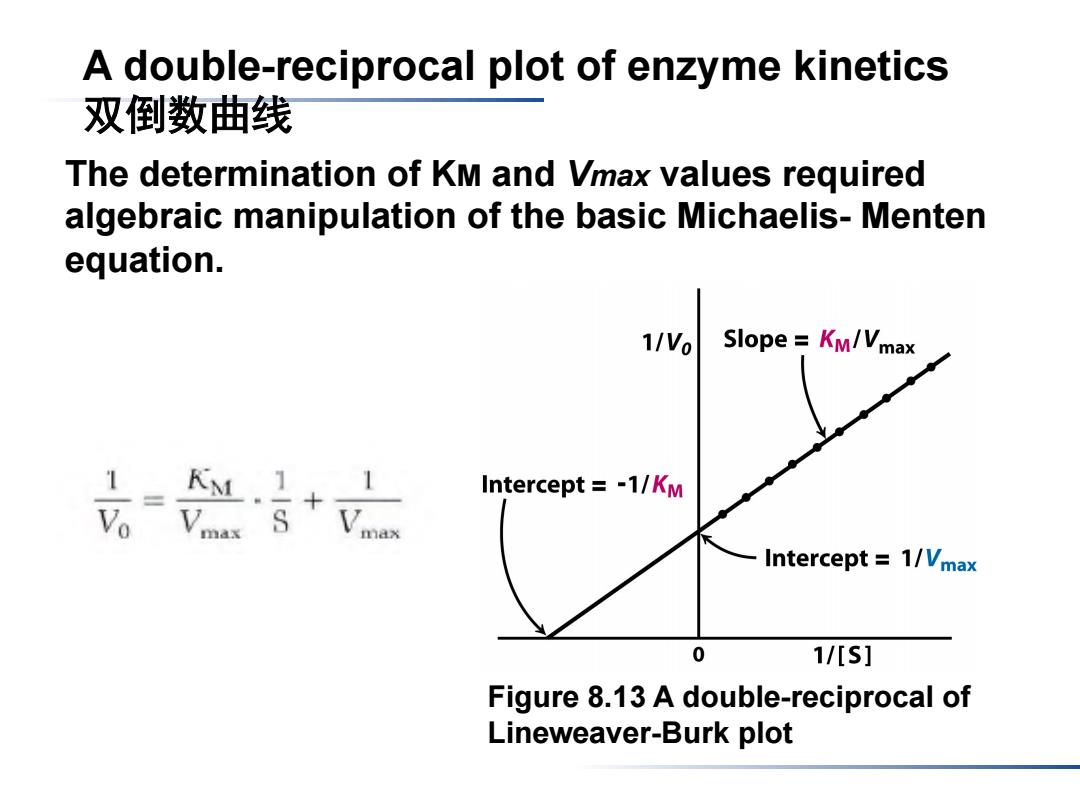
A double-reciprocal plot of enzyme kinetics 双倒数曲线 The determination of KM and Vmax values required algebraic manipulation of the basic Michaelis-Menten equation. 1/Vo Slope Km/Vmax 1 KM Intercept =-1/KM Intercept =1/Vmax 0 1/[s] Figure 8.13 A double-reciprocal of Lineweaver-Burk plot
Figure 8.13 A double-reciprocal of Lineweaver-Burk plot The determination of KM and Vmax values required algebraic manipulation of the basic Michaelis- Menten equation. A double-reciprocal plot of enzyme kinetics 双倒数曲线
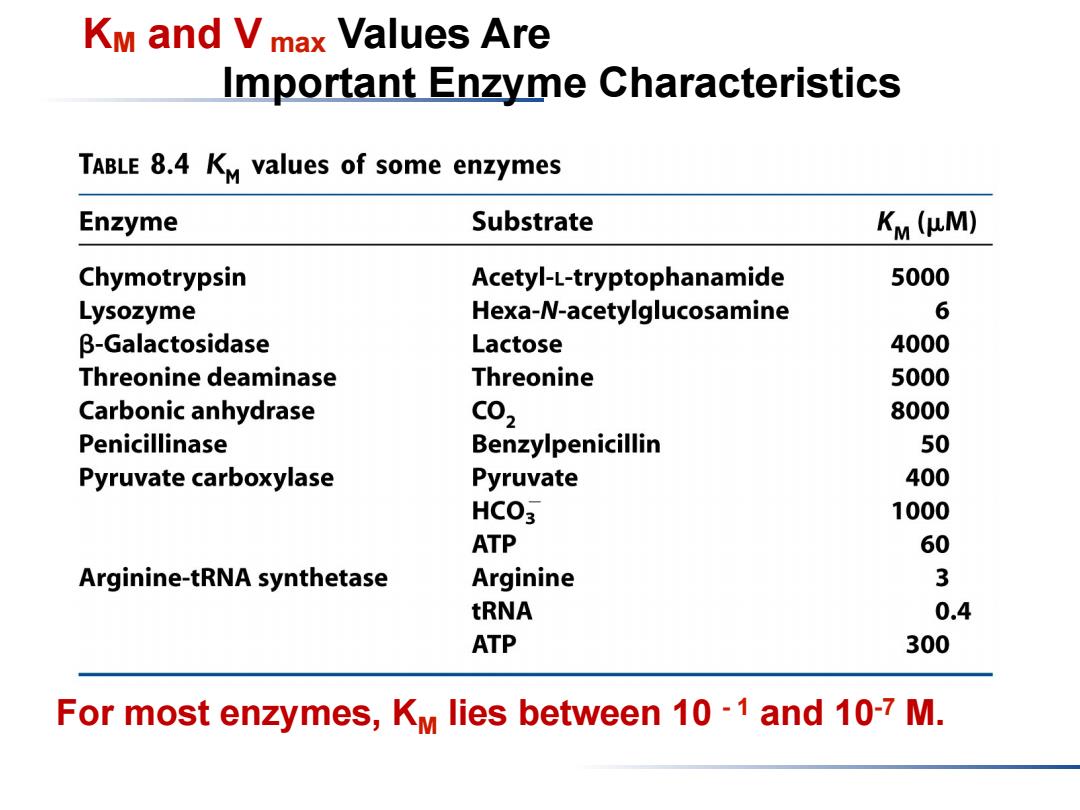
KM and Vmax Values Are Important Enzyme Characteristics TABLE 8.4 KM values of some enzymes Enzyme Substrate Km (HM) Chymotrypsin Acetyl-L-tryptophanamide 5000 Lysozyme Hexa-N-acetylglucosamine 6 β-Galactosidase Lactose 4000 Threonine deaminase Threonine 5000 Carbonic anhydrase C02 8000 Penicillinase Benzylpenicillin 50 Pyruvate carboxylase Pyruvate 400 HCO3 1000 ATP 60 Arginine-tRNA synthetase Arginine 3 tRNA 0.4 ATP 300 For most enzymes,KM lies between 10-1 and 10-7 M
KM and V max Values Are Important Enzyme Characteristics For most enzymes, KM lies between 10 - 1 and 10-7 M

Km is the concentration of substrate at which half the active sites are filled.Thus,Km provides a measure of the substrate concentration required for significant catalysis to take place. 因为KM值是酶活性部位填充一半时底物浓度,所以可以提 供了测定底物浓度的手段。 For many enzymes,experimental evidence suggests that Km provides an approximation of substrate concentration in vivo. 对很多酶来讲,KM提供了体内可能的底物浓度
KM is the concentration of substrate at which half the active sites are filled. Thus, KM provides a measure of the substrate concentration required for significant catalysis to take place. 因为KM值是酶活性部位填充一半时底物浓度,所以可以提 供了测定底物浓度的手段。 For many enzymes, experimental evidence suggests that KM provides an approximation of substrate concentration in vivo. 对很多酶来讲, KM 提供了体内可能的底物浓度
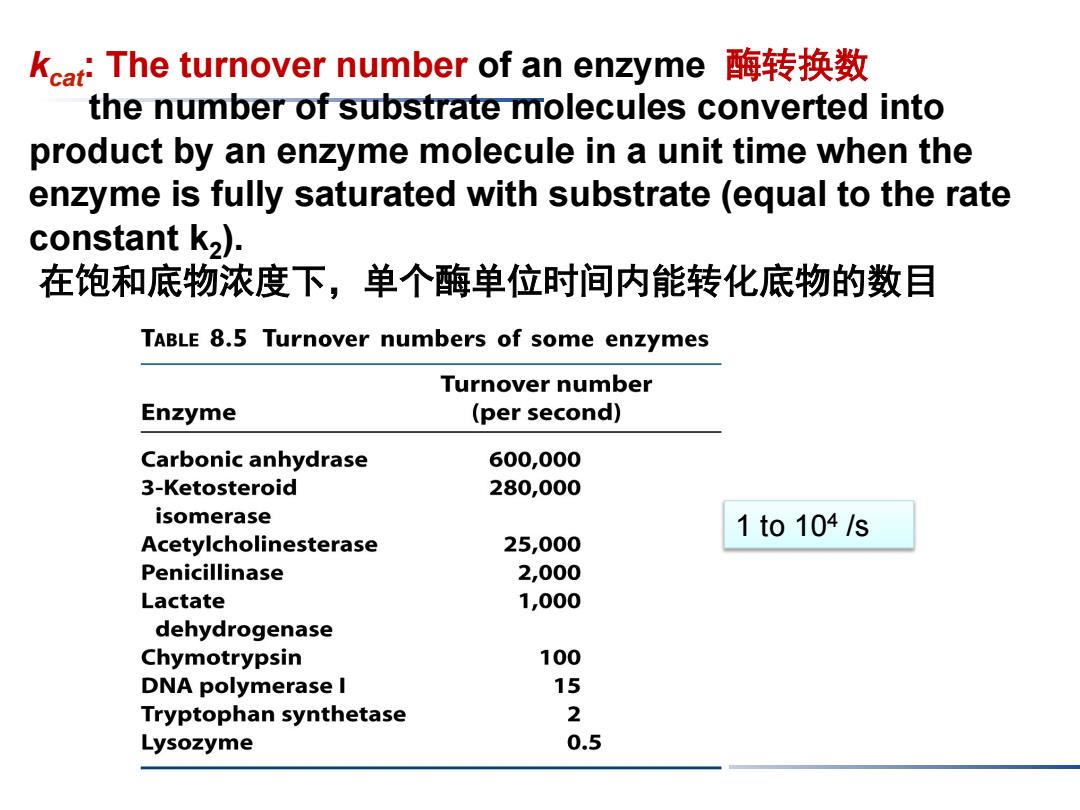
kcat:The turnover number of an enzyme酶转换数 the number of substrate molecules converted into product by an enzyme molecule in a unit time when the enzyme is fully saturated with substrate(equal to the rate constant k,). 在饱和底物浓度下,单个酶单位时间内能转化底物的数目 TABLE 8.5 Turnover numbers of some enzymes Turnover number Enzyme (per second) Carbonic anhydrase 600,000 3-Ketosteroid 280,000 isomerase 1to104/s Acetylcholinesterase 25,000 Penicillinase 2,000 Lactate 1,000 dehydrogenase Chymotrypsin 100 DNA polymerase I 15 Tryptophan synthetase 2 Lysozyme 0.5
1 to 104 /s kcat: The turnover number of an enzyme 酶转换数 the number of substrate molecules converted into product by an enzyme molecule in a unit time when the enzyme is fully saturated with substrate (equal to the rate constant k2). 在饱和底物浓度下,单个酶单位时间内能转化底物的数目