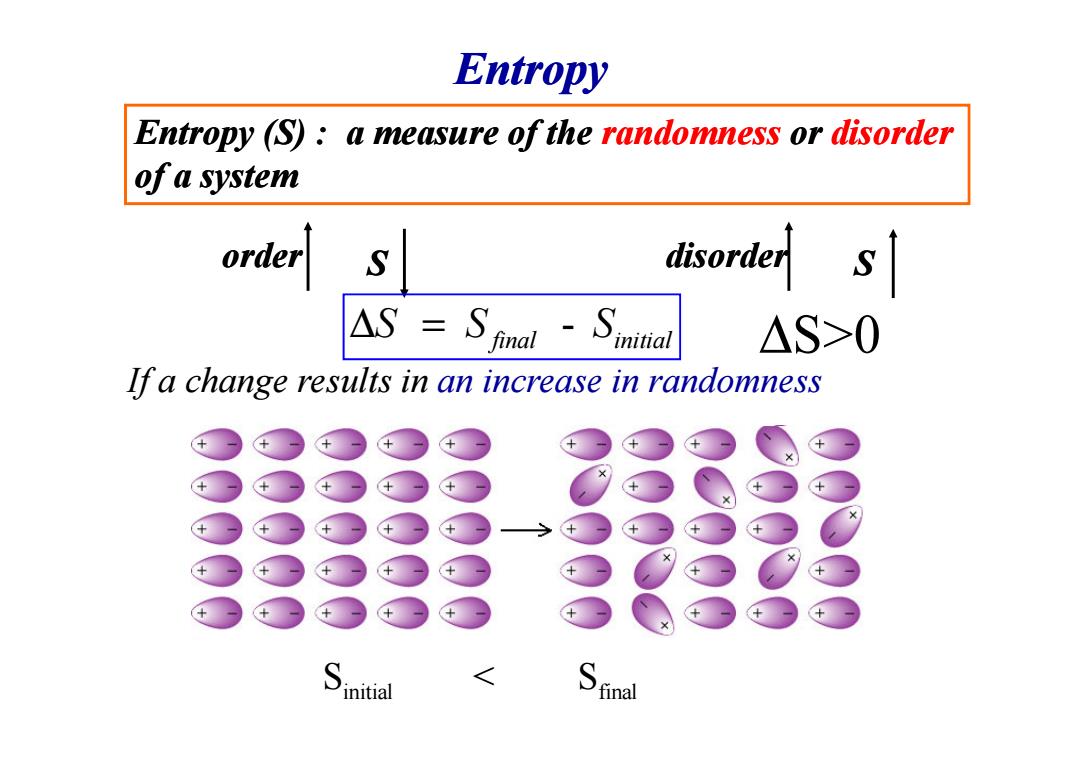
Entropy Entropy (S):a measure of the randomness or disorder of a system order S △S>0 If a change results in an increase in randomness initial S inal
Entropy (S) : a measure of the randomness or disorder of a system order S disorder S If a change results in an increase in randomness Entropy - final initial ∆ = S S S ∆S>0 If a change results in an increase in randomness initial final S < S

Entropy,S Entropy a thermodynamic quantity,similar to enthalpy ·A state.function: AS=Sfinat -Stmimal the change of entropy (AS)depends only on the initial and final states of the system not on the path it takes to change states An extensive property its value depends on the quantity (mass,mole)of a substance. S(of 1mole O2(g))<S(of 2 mole O2(g))
Entropy, S Entropy : a thermodynamic quantity, similar to enthalpy • A state function: – the change of entropy (∆S) depends • only on the initial and final states of the system - final initial ∆ = S S S • not on the path it takes to change states • An extensive property : its value depends on the quantity (mass, mole) of a substance. 2 2 S(of 1mole O (g)) < S(of 2 mole O (g))

Entropy Boltzman the entropy (S)of a system is related to the natural log of the number of microstates (W). S=k.InW AS=S final-Similial AS=S inal -S'initial =k In Winal -k In Wininal =kIn W itial WFmal >Winital,AS> WEmal <Wial.AS<
Boltzman : the entropy (S) of a system is related to the natural log of the number of microstates (W). Entropy final initial S k = ⋅lnW ∆ = − S S S ln ln ln final initial final final initial initial S S S W k W k W k W ∆ = − = − = , 0 W W S Final initial > ∆ > , 0 W W S Final initial < ∆ <

Ludwig Boltzmann s-k fog w An Austrian physicist famous for his founding contribution in the fields of statistical mechanics and statistical thermodynamics EVDIG BOLIZMANN DPILFAULA BOLTZMANN 5 鞋 1 S=k·lnW BOLEZNNN OCTZMANK BOUZMANN Among his students in Graz were Svante Arrhenius and Walther Nernst
An Austrian physicist famous for his founding contribution in the Ludwig Boltzmann Nov. 2008 S k = ⋅ln W his founding contribution in the fields of statistical mechanics and statistical thermodynamics Among his students in Graz were Svante Arrhenius and Walther Nernst

Entropy ep.Four Molecules in a Two-Bulbed Flask S=k.InW W:the possible arrangements (states) Arrangement I Microstates three Possible 0 Arrangements W=3 Arrangement II k=R/N=8.314J/mol.K/6.02×1023mol-1 =1.38×10-23J/K S=k.InW=1.38x1023J/KxIn3 Arrangement III =1.516×10-23J/K Probability and the locations of gas molecules
Microstates : three Possible Arrangements S k = ⋅lnW Entropy W=3 ep. :Four Molecules in a Two in a Two-Bulbed Flask Bulbed Flask W: the possible arrangements (states) Arrangements 23 S k J K ln 1.38 10 ln W / 3 − = ⋅ = × × 23 1 23 8.314 / 6.02 10 = 1.38 10 / k R N J mol K mol J K − − = = ⋅ × × W=3 23 1.516 10 / J K − = × Probability and the locations of gas molecules