
The principle of multiple equilibria K9=(K9)2k8=0.672×0.051=0.023 Conclusion: if a reaction equation is obtained by a linear combination of a couple of reaction equations,the reaction equilibrium constant of the resulting combined reaction is equal to the product /or quotient of the participating reactions'reaction equilibrium constant. ------a principle of multi-equilibria 结论:如果多个反应的计量式经过线性组合得到一个总的化学计量式,则总反应 的标准平衡常数等于各反应的平衡常数之积或商—多重平衡原理
The principle of multiple equilibria Conclusion: if a reaction equation is obtained by a linear combination of a couple of reaction equations, the reaction equilibrium constant of the resulting combined reaction is equal to the product /or quotient of the participating reactions’ reaction equilibrium constant. ------a principle of multi-equilibria 结论: 如果多个反应的计量式经过线性组合得到一个总的化学计量式,则总反应 的标准平衡常数等于各反应的平衡常数之积或商⎯⎯多重平衡原理. = · = 0.67 K 3 K 2 ×0.051=0.023 1 K 2 2 ( )

4.1.3 Experimental measurement of ke Example:GeO(g)and W2O(g)can react to give GeWO(g)at high temperature 2Ge0(g)+W,06(g)=2GeWO4(g) The reaction starts with a mixture of GeO and W2O, both at pressures 100.0kPa in a constant volume vessel at a certain temperature.Analysis shows that the partial pressure of GeWO4(g)is 98.0kPa at equilibrium. Calculate the partial pressures of GeO and W2O at equilibrium and K for the reaction
Example:GeO(g) and W2O6 (g) can react to give GeWO4 (g) at high temperature 4.1.3 Experimental measurement of The reaction starts with a mixture of GeO and W2O6, both at pressures 100.0kPa in a constant volume vessel at a certain temperature. Analysis shows that the partial pressure of GeWO4 (g) is 98.0kPa at equilibrium. Calculate the partial pressures of GeO and W2O6 at equilibrium and KΘ for the reaction. 2GeO (g) +W2O6 (g) 2 GeWO4 (g) K
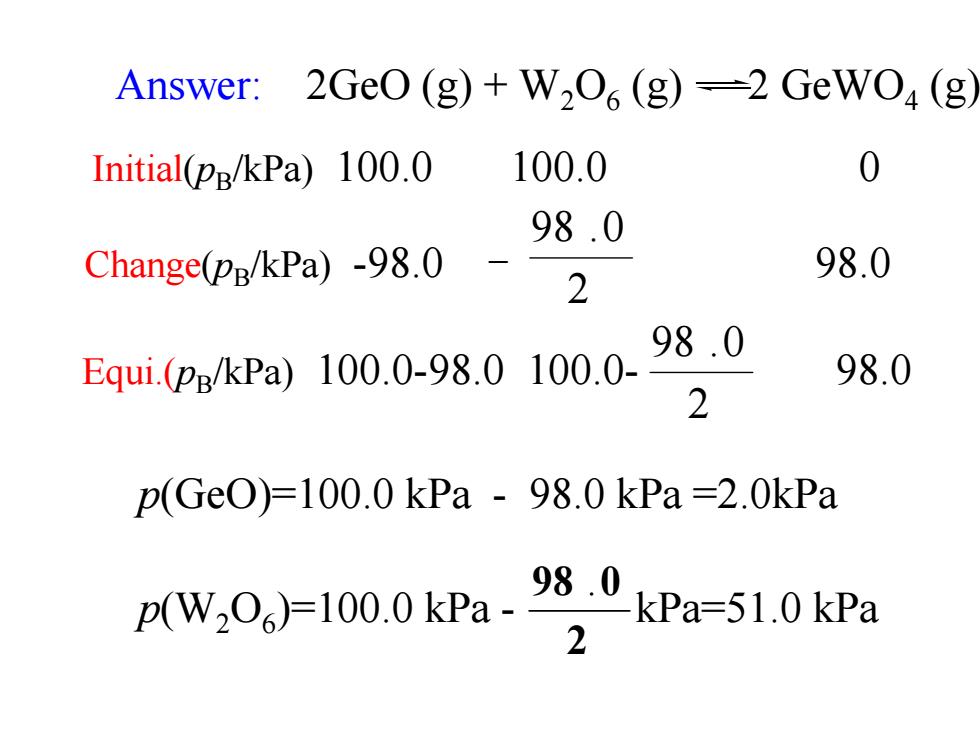
Answer:2GeO(g)+W2O(g)-2 GeWOa(g Initial(pp/kPa)100.0 100.0 0 98.0 Change(pg/kPa)-98.0 98.0 2 98.0 Equi.(pB/kPa)100.0-98.0100.0- 98.0 2 p(Ge0)=100.0kPa-98.0kPa=2.0kPa pW,0,100kPa.98,0ka=510kPa 2
2 98 .0 p(W2O6)=100.0 kPa - kPa=51.0 kPa p(GeO)=100.0 kPa - 98.0 kPa =2.0kPa Answer: 2GeO (g) + W2O6 (g) 2 GeWO4 (g) 2 98 .0 Equi.(pB/kPa) 100.0-98.0 100.0- 98.0 Initial(pB/kPa) 100.0 100.0 0 Change(pB/kPa) -98.0 - 98.0 2 98 .0

K8= [p(Gewo )pe 12 [p(Geo)/pe P[p(W2O)/pe (98.0/1002 =4.7×103 (2.0/100)2(51.0/100)
( ) ( ) ( ) 3 2 2 4.7 10 2.0 100 51.0 100 98.0 100 = = × ( ) [ () ( ) GeO / ] [ W O / ] [ GeWO / ] 2 6 2 2 4 p p p p p p K =
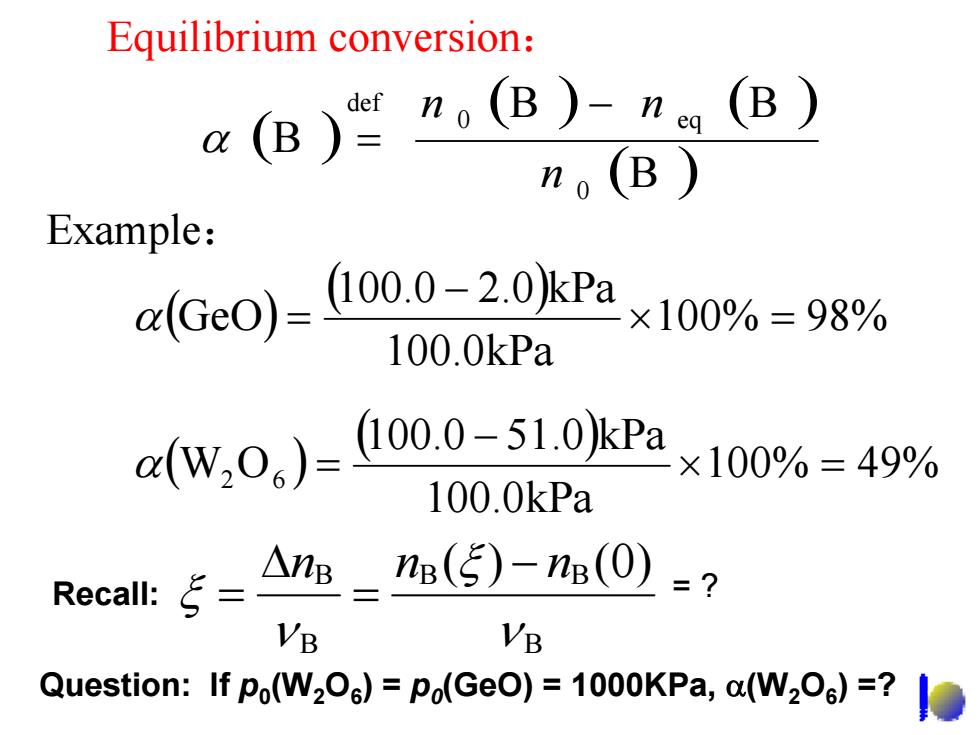
Equilibrium conversion: a(B)”。(B)-n (B) ) Example: x4Ge0))=L000-20kPax10%=98% 100.0kPa a(w,0)=10.0-51.0kPa 100%=49% 100.0kPa Recall:= △ne-n(5)-n(0) =7 VB VB Question:If po(W2Oe)=po(GeO)=1000KPa,a(W2Os)=?
Equilibrium conversion : ( ) ( ) ( ) ( ) B B B B 0 0 eq def n n − n α = ( ) ( ) 100 % 49 % 100.0kPa 100.0 51.0 kPa W 2 O 6 × = − α = ( ) ( ) 100 % 98 % 100.0kPa 100.0 2.0 kPa GeO × = − α = Example : Question: If p 0(W 2 O 6) = p 0(GeO) = 1000KPa, α(W 2 O 6) =? = ? B B B B B ( ) ( 0 ) ν ξ ν ξ n n − n = Δ Recall: =