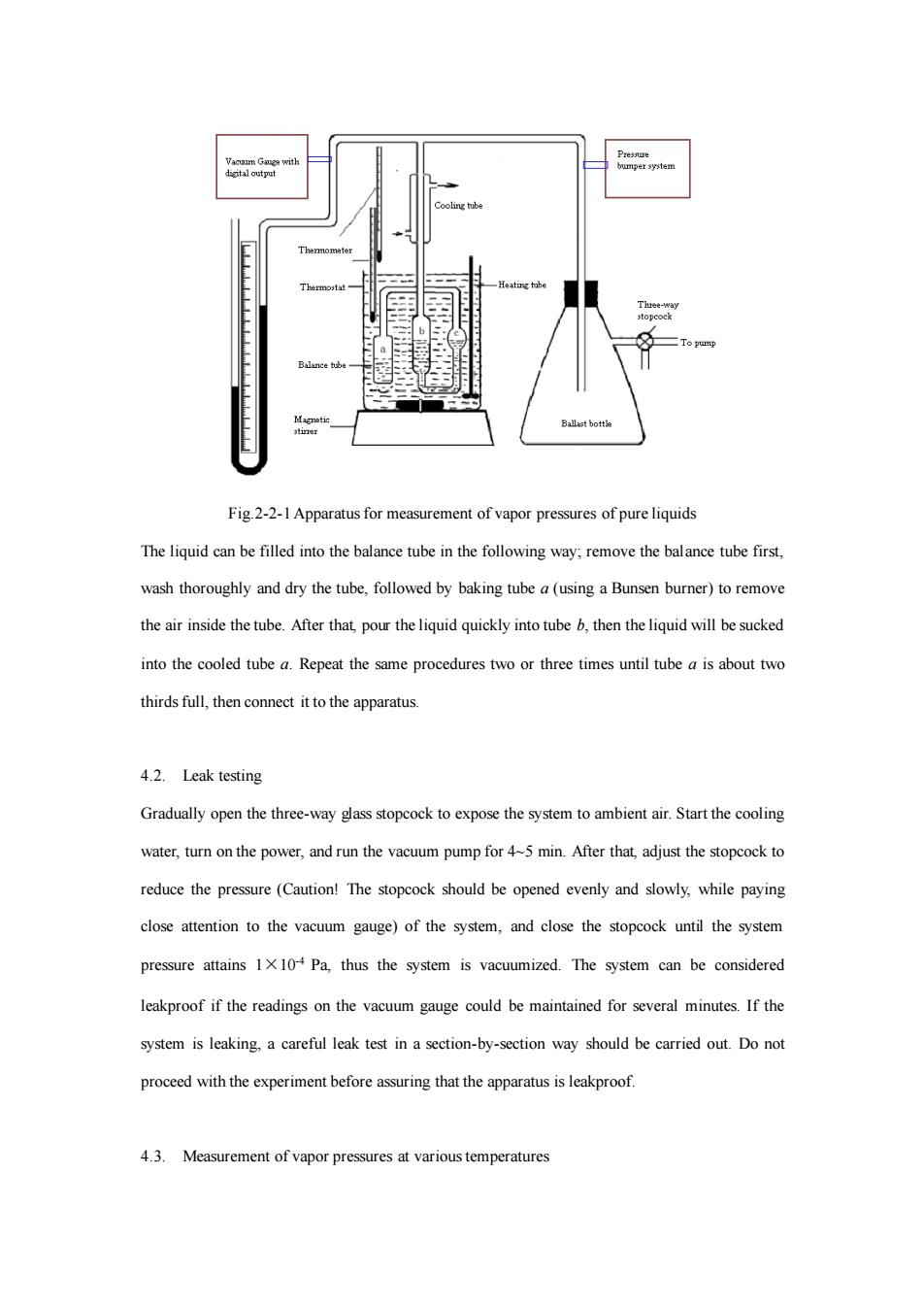
Fig 2-2-1 Apparatus for measurement of vapor pressures of pure liquids The liquid can be filled into the balance tube in the following way:remove the balance tube first. wash thoroughly and dry the tube,followed by baking tube a(using a Bunsen burner)to remove the air inside the tube.After that pour the liquid quickly into tube then the liquid will be sucked into the cooed tube a.Repeat the same procedures two or three times until tube is about two thirds full,then conect it to the apparatus. 4.2.Leak testing Gradually open the three-way ass stopcock to expo the system toambient air.Start the cooin water,turn on the power,and run the vacuum pump for45 min.After that adjust the stopcock to reduce the pressure (Caution!The stopcock should be opened evenly and slowly,while paying close attention to the vacuum gauge)of the system,and the stopcock until the system pressure attains 1x10 Pa,thus the system is vacuumized.The system can be considered eakproof if the readings on the vacuum gauge cou be maintained for several minutes If the system is leaking.a careful leak test in a section-by-section way should be carried out.Do not proceed with the experiment before assuring that the apparatus is leakproof 4.3.Measurement of vapor pressures at various temperatures
Fig.2-2-1 Apparatus for measurement of vapor pressures of pure liquids The liquid can be filled into the balance tube in the following way; remove the balance tube first, wash thoroughly and dry the tube, followed by baking tube a (using a Bunsen burner) to remove the air inside the tube. After that, pour the liquid quickly into tube b, then the liquid will be sucked into the cooled tube a. Repeat the same procedures two or three times until tube a is about two thirds full, then connect it to the apparatus. 4.2. Leak testing Gradually open the three-way glass stopcock to expose the system to ambient air. Start the cooling water, turn on the power, and run the vacuum pump for 4~5 min. After that, adjust the stopcock to reduce the pressure (Caution! The stopcock should be opened evenly and slowly, while paying close attention to the vacuum gauge) of the system, and close the stopcock until the system pressure attains 1×10-4 Pa, thus the system is vacuumized. The system can be considered leakproof if the readings on the vacuum gauge could be maintained for several minutes. If the system is leaking, a careful leak test in a section-by-section way should be carried out. Do not proceed with the experiment before assuring that the apparatus is leakproof. 4.3. Measurement of vapor pressures at various temperatures

Open the system to ambient by turning the three-way stopcock.Turn on the stirrer and heat up the water bath.As the temperature increases,the liquid in the balance tube will bubble up.Keep heating the system to a temperature of about 5C above the normal boiling point of the liquid Maintain this temperature for several minutes to remove al remained air in the balance tube 4.3.1 Measuring the billing point at ambient pressure 4.3.2.Measuring vapor pressures of pure liquid at various temperatures 1.Data Analysis 5.1 Design a table for the experimental results,and accurately report both the original data and the calculated results 5.2 Accurate measurement of temperature is critical for this experiment The thermometer should be calibrated for the exposed stem as described in Chapter 1 of Part Three. .3 Polt vapor pressure pagainst temperatureT. Experiment 1 Molar Mass from Depression of Freezing Point 2.Objective 1.1.To gain further insights into the colligatives of dilutesoutions 1.2.To learn the experimental technique for freezing point measurement 2.3.To determine the molar mass of naphthalene by measuring the depression of freezing point 3.Introduction The freezing point of a solution is the temperature at which the solid solvent exists in equilibrium with the liquid solution.When an involatilesolute is added toa sovent to make a bi-componen dilute solution,it lowers the solvent's freezing point.The depression is a colligative property of a dilute solution,because it depends only on the number of molecules of the solute if the nature and amount of the solvent are fixed.For an ideal solution,according to the phase equilibrium condition,the relationship of the freezing point depression to the components ofadilute olution
Open the system to ambient by turning the three-way stopcock. Turn on the stirrer and heat up the water bath. As the temperature increases, the liquid in the balance tube will bubble up. Keep heating the system to a temperature of about 5℃ above the normal boiling point of the liquid. Maintain this temperature for several minutes to remove all remained air in the balance tube. 4.3.1 Measuring the billing point at ambient pressure 4.3.2. Measuring vapor pressures of pure liquid at various temperatures 1. Data Analysis 5.1 Design a table for the experimental results, and accurately report both the original data and the calculated results. 5.2 Accurate measurement of temperature is critical for this experiment. The thermometer should be calibrated for the exposed stem as described in Chapter 1 of Part Three. 5.3 Polt vapor pressure p* against temperature T. Experiment 1 Molar Mass from Depression of Freezing Point 2. Objective 1.1. To gain further insights into the colligatives of dilute solutions 1.2. To learn the experimental technique for freezing point measurement 2.3. To determine the molar mass of naphthalene by measuring the depression of freezing point 3. Introduction The freezing point of a solution is the temperature at which the solid solvent exists in equilibrium with the liquid solution. When an involatile solute is added to a solvent to make a bi-component dilute solution, it lowers the solvent’s freezing point. The depression is a colligative property of a dilute solution, because it depends only on the number of molecules of the solute if the nature and amount of the solvent are fixed. For an ideal solution, according to the phase equilibrium condition, the relationship of the freezing point depression to the components of a dilute solution

can be expressed by the van't Hoff freezing point depression equation R(T)na △T,=AH(Am+g where AT is the depression of freezing point;T is the freezing point for the pure solvent; A()is the molar enthalpy of fusion of the solvent:mnd mare the number of moles of the solvent and solute,respectively. For a dilutesolution,the nuber of moles of the much greater than that of the solute thus 、RT》2aR(T} a灯-a6ay0xMm=km where M is the solute's molar mass.is the molality of the the cryogenic constant of the solvent. If the cryogenic constant Kyof a solvent is known,and the depression of the freezing point(AT) and the masses of solvent and solute(Wand )have been measured,then the molar mass of the solute can be found. 4.Apparatus and Reagent Freezing point apparatus;Beckmann thermometer with digital output;Mercury thermometer; Pipette:1000 mL beaker;Pellet press.Cyclohexane(AR):Naphthalene (AR):Crushed ice. 5.Procedure 4.1.Assemble the apparatus Assemble the freezing point apparatus as Fig2-1-2.Make sure that the freezing tube,the probe of the Beckmann thermometer with digital output and the stirrer are perfectly clean and dry,and the stirrer moves freely without contacting the inner wall of the freezing tube or the thermometer. 4.2.Adjust the temperature of the freezing agent
can be expressed by the van’t Hoff freezing point depression equation A B B f m f f n n n H A R T T + = ( ) ( ) * 2 where Tf is the depression of freezing point; * Tf is the freezing point for the pure solvent; H (A) f m is the molar enthalpy of fusion of the solvent; nA and nB are the number of moles of the solvent and solute, respectively. For a dilute solution, the nuber of moles of the solvent nA is much greater than that of the solute nB, thus A B f B f m f A B f m f f M m K m H A R T n n H A R T T = = = ( ) ( ) ( ) ( ) * 2 * 2 where MA is the solute’s molar mass, mB is the molality of the solute; Kf is the cryogenic constant of the solvent. If the cryogenic constant Kf of a solvent is known, and the depression of the freezing point ( Tf ) and the masses of solvent and solute (WA and WB) have been measured, then the molar mass of the solute can be found. 4. Apparatus and Reagent Freezing point apparatus; Beckmann thermometer with digital output; Mercury thermometer; Pipette; 1000 mL beaker; Pellet press; Cyclohexane (AR); Naphthalene (AR); Crushed ice. 5. Procedure 4.1. Assemble the apparatus Assemble the freezing point apparatus as Fig.2-1-2. Make sure that the freezing tube, the probe of the Beckmann thermometer with digital output and the stirrer are perfectly clean and dry, and the stirrer moves freely without contacting the inner wall of the freezing tube or the thermometer. 4.2. Adjust the temperature of the freezing agent

Adjust the amount of crushed ice and water to set the temperature of the freezing agent at c.3.5C (namely,to maintain the temperature of the ice bath within 3C below the freezing point of the ).Stirthe freezing agent frequently and add crushed ice if necessary. 4.3.Measure the freezing point of the solvent Quantitatively transfer 25 mL of cyclohexane into the freezing tube using a pipette The amount of cyclohexane should be enough to immerse the probe of the Beckmann thermometer. Meanwhile,try to avoid the added cyclohexane splashing onto the inner wall of the freezing tube. Stopper the freezing tube with a cork to prevent evaporation of cyclohexane.Take down the temperature of the Immerse the freezing tube containing yclohexane into the freezing agent directly.stir uniformly and continuously with an up and down movement to cool the solvent gradually. Remove the freezing tube from the apparatus when solid begins to form in the liquid.Wipe the outside of the freezing tube dry,and insert it into the air-jacket.Stir the liquid slowly and continuously with an up and down movement (at a speed of about once per second).Read the Beckmann thermometer till the temperature keeps stable.This is the approximate freezing point of cyclohexane. Remove the freezing tube from the apparatus and melt the cyclohexane by resting the tube in the open palm of you hand Then,replace the tube into the freezing agent and stir slowly to cool down the solvent more rapidly.Remove the freezing tube quickly when the temperature of the solvent decreases to ca.5C above the approximate freezing point,wipe the outside of the freezing tube dry.insert it into the air-jacket and stir the solvent slowly and continuously with an up and down movement(at a speed of about once per second)to cool down the temperature of cyclohexane.Stir the solvent vigorously when the temperature drops to0.3c below the freezing point to accelerate the crystallization of cyelohexane avoiding supercooling of the liquid by more than 0.5C.Upon crystallization of cyclohexane,the temperature of the system starts to increase.Then promptly slow down the stirring rate,and record continuously the reading of the
Adjust the amount of crushed ice and water to set the temperature of the freezing agent at c. 3.5℃ (namely, to maintain the temperature of the ice bath within 3℃ below the freezing point of the solution). Stir the freezing agent frequently and add crushed ice if necessary. 4.3. Measure the freezing point of the solvent Quantitatively transfer 25 mL of cyclohexane into the freezing tube using a pipette. The amount of cyclohexane should be enough to immerse the probe of the Beckmann thermometer. Meanwhile, try to avoid the added cyclohexane splashing onto the inner wall of the freezing tube. Stopper the freezing tube with a cork to prevent evaporation of cyclohexane. Take down the temperature of the solvent. Immerse the freezing tube containing cyclohexane into the freezing agent directly; stir uniformly and continuously with an up and down movement to cool the solvent gradually. Remove the freezing tube from the apparatus when solid begins to form in the liquid. Wipe the outside of the freezing tube dry, and insert it into the air-jacket. Stir the liquid slowly and continuously with an up and down movement (at a speed of about once per second). Read the Beckmann thermometer till the temperature keeps stable. This is the approximate freezing point of cyclohexane. Remove the freezing tube from the apparatus and melt the cyclohexane by resting the tube in the open palm of your hand. Then, replace the tube into the freezing agent and stir slowly to cool down the solvent more rapidly. Remove the freezing tube quickly when the temperature of the solvent decreases to ca. 0.5℃ above the approximate freezing point, wipe the outside of the freezing tube dry, insert it into the air-jacket and stir the solvent slowly and continuously with an up and down movement (at a speed of about once per second) to cool down the temperature of cyclohexane. Stir the solvent vigorously when the temperature drops to 0.2~0.3℃ below the freezing point to accelerate the crystallization of cyclohexane avoiding supercooling of the liquid by more than 0.5℃. Upon crystallization of cyclohexane, the temperature of the system starts to increase. Then promptly slow down the stirring rate, and record continuously the reading of the

Beckmann thermometer till it keeps stable.The stable reading is the freezing point of cyclohexane. Repeat the freezing point determination three times.The readings should agree within003C. 4.4.Measure the freezing point of the solution Remove the freezing tube from the apparatus and melt the crystallized cyclohexane.Drop one of the precisely weighed naphthalene pellets (the amount of naphthalene could approximately depress the freezing point of the solution by about 5C)into the freezing tube through the side arm.The procedure for freezing point determination of is the same as that for the pure solvent,ie.to find out the approximate freezing point at first and to measure the precise value subsequently.The maximum temperature of the solution observed after supercooling is the freezing point.Repeat the freezing point determination three times The readings should agree within±0.003C. 5.Data Analysis 5.1.Calculate the density of cyclohexane at room temperature employinggcm)= 0.7971-0.8879103/C,and then calculate the mass W of cyclohexane used in this experiment. 5.2.Calculate the molar of naphthalene from the measured freezing point data,T and T for pure solvent and solution,respectively,and estimate the possible molecular state of naphthalene dissolved in yclohexane
Beckmann thermometer till it keeps stable. The stable reading is the freezing point of cyclohexane. Repeat the freezing point determination three times. The readings should agree within ±0.003℃. 4.4. Measure the freezing point of the solution Remove the freezing tube from the apparatus and melt the crystallized cyclohexane. Drop one of the precisely weighed naphthalene pellets (the amount of naphthalene could approximately depress the freezing point of the solution by about 0.5℃) into the freezing tube through the side arm. The procedure for freezing point determination of the solution is the same as that for the pure solvent, i.e. to find out the approximate freezing point at first and to measure the precise value subsequently. The maximum temperature of the solution observed after supercooling is the freezing point. Repeat the freezing point determination three times. The readings should agree within ±0.003℃. 5. Data Analysis 5.1. Calculate the density of cyclohexane at room temperature t employing ρt/(g·cm-3 ) = 0.7971-0.8879×10-3 t/℃, and then calculate the mass WA of cyclohexane used in this experiment. 5.2. Calculate the molar of naphthalene from the measured freezing point data, * Tf and Tf for pure solvent and solution, respectively, and estimate the possible molecular state of naphthalene dissolved in cyclohexane