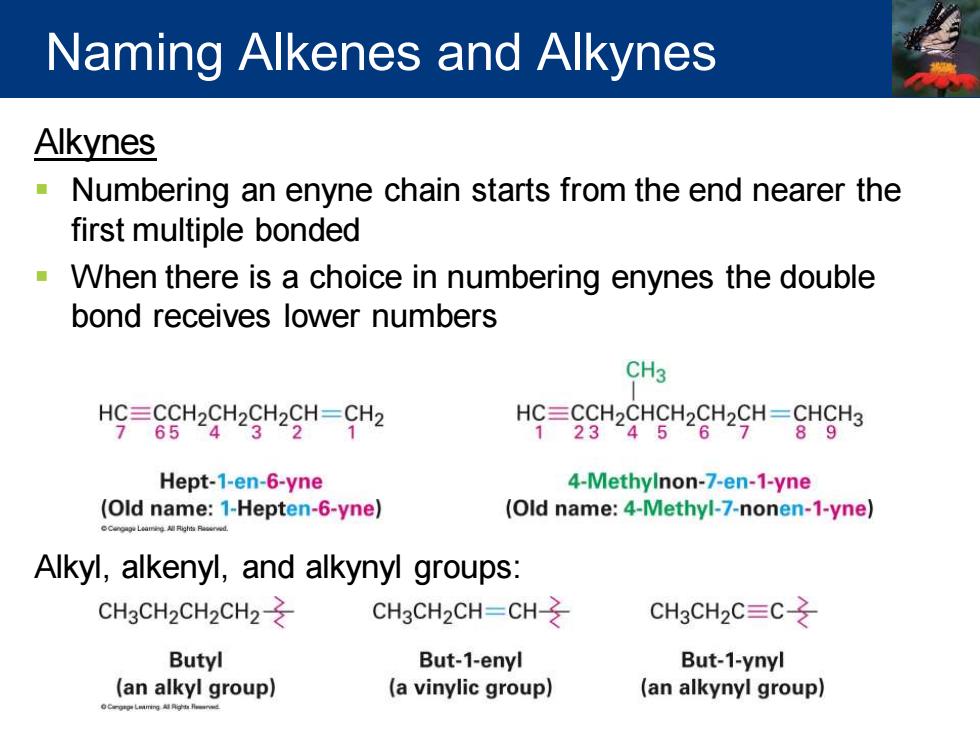
Naming Alkenes and Alkynes Alkynes Numbering an enyne chain starts from the end nearer the first multiple bonded When there is a choice in numbering enynes the double bond receives lower numbers CH3 HC=CCH2CH2CH2CH-CH2 HC=CCH2CHCH2CH2CH=CHCH3 765243“21 23456789 Hept-1-en-6-yne 4-Methylnon-7-en-1-yne (Old name:1-Hepten-6-yne) (Old name:4-Methyl-7-nonen-1-yne) Alkyl,alkenyl,and alkynyl groups: CH3CH2CH2CH2- CH3CH2CH=CH- CH3CH2C=C Butyl But-1-enyl But-1-ynyl (an alkyl group) (a vinylic group) (an alkynyl group)
Alkynes ▪ Numbering an enyne chain starts from the end nearer the first multiple bonded ▪ When there is a choice in numbering enynes the double bond receives lower numbers Alkyl, alkenyl, and alkynyl groups: Naming Alkenes and Alkynes

7-3 Cis-Trans lsomerism in Alkenes Carbon-carbon double bond description -Valence bond language Carbons are sp2 hybridized Three equivalent hybrid orbitals that lie in a plane at angles of 120°to one another Carbons form a o bond by head-on overlap of sp2 orbitals and a p bond by sideways overlap of unhybridized p orbitals oriented perpendicular to sp2 plane p orbitals. bond sp2orbitals bond sp2carbon sp2 carbon Carbon-carbon double bond 121.3 108.7pm 134pm
7-3 Cis-Trans Isomerism in Alkenes Carbon-carbon double bond description ▪Valence bond language ▪ Carbons are sp2 hybridized ▪ Three equivalent hybrid orbitals that lie in a plane at angles of 120º to one another ▪ Carbons form a σ bond by head-on overlap of sp2 orbitals and a p bond by sideways overlap of unhybridized p orbitals oriented perpendicular to sp2 plane
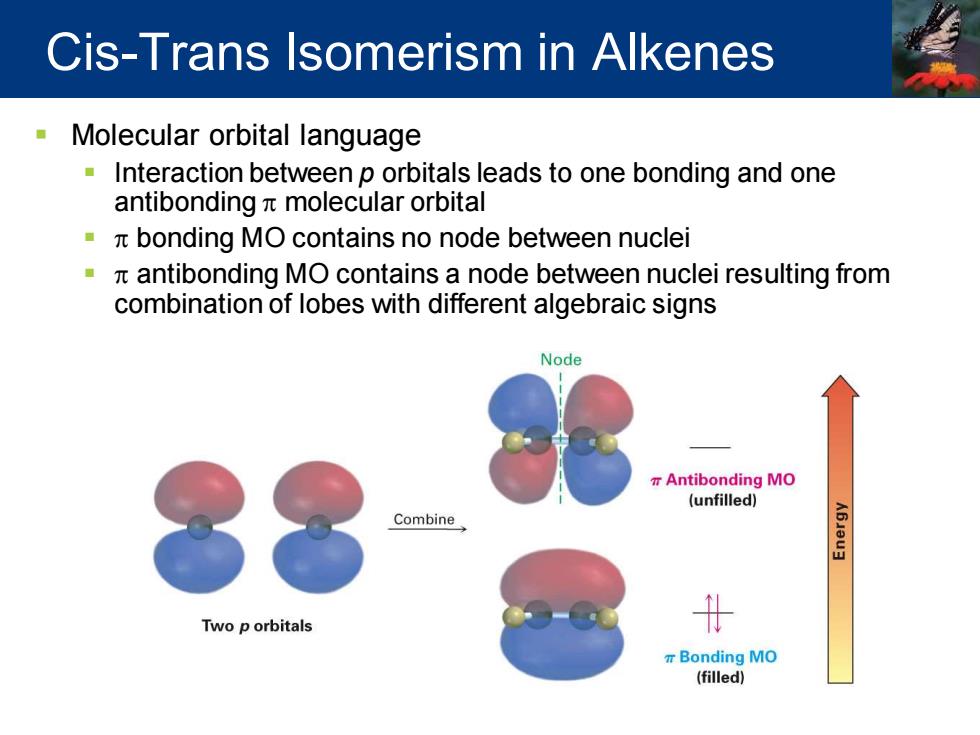
Cis-Trans Isomerism in Alkenes Molecular orbital language Interaction between p orbitals leads to one bonding and one antibonding nt molecular orbital bonding MO contains no node between nuclei antibonding MO contains a node between nuclei resulting from combination of lobes with different algebraic signs Node Antibonding MO (unfilled) Combine Two p orbitals t r Bonding MO (filled)
▪ Molecular orbital language ▪ Interaction between p orbitals leads to one bonding and one antibonding p molecular orbital ▪ p bonding MO contains no node between nuclei ▪ p antibonding MO contains a node between nuclei resulting from combination of lobes with different algebraic signs Cis-Trans Isomerism in Alkenes
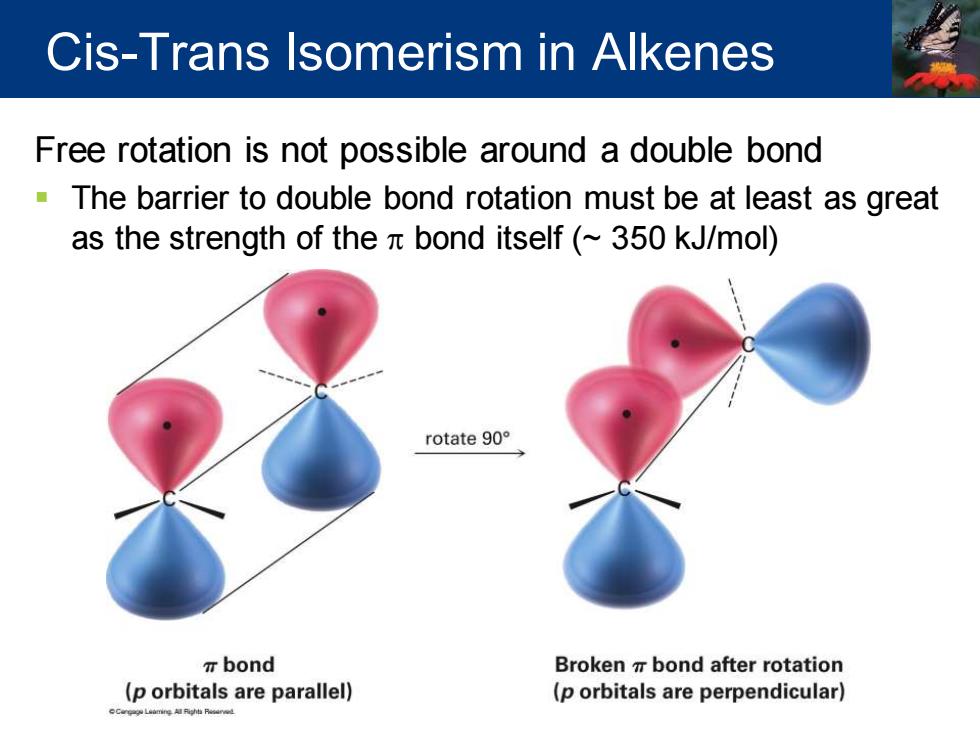
Cis-Trans Isomerism in Alkenes Free rotation is not possible around a double bond -The barrier to double bond rotation must be at least as great as the strength of the bond itself(~350 kJ/mol) rotate90° T bond Broken bond after rotation (p orbitals are parallel) (p orbitals are perpendicular)
Free rotation is not possible around a double bond ▪ The barrier to double bond rotation must be at least as great as the strength of the p bond itself (~ 350 kJ/mol) Cis-Trans Isomerism in Alkenes
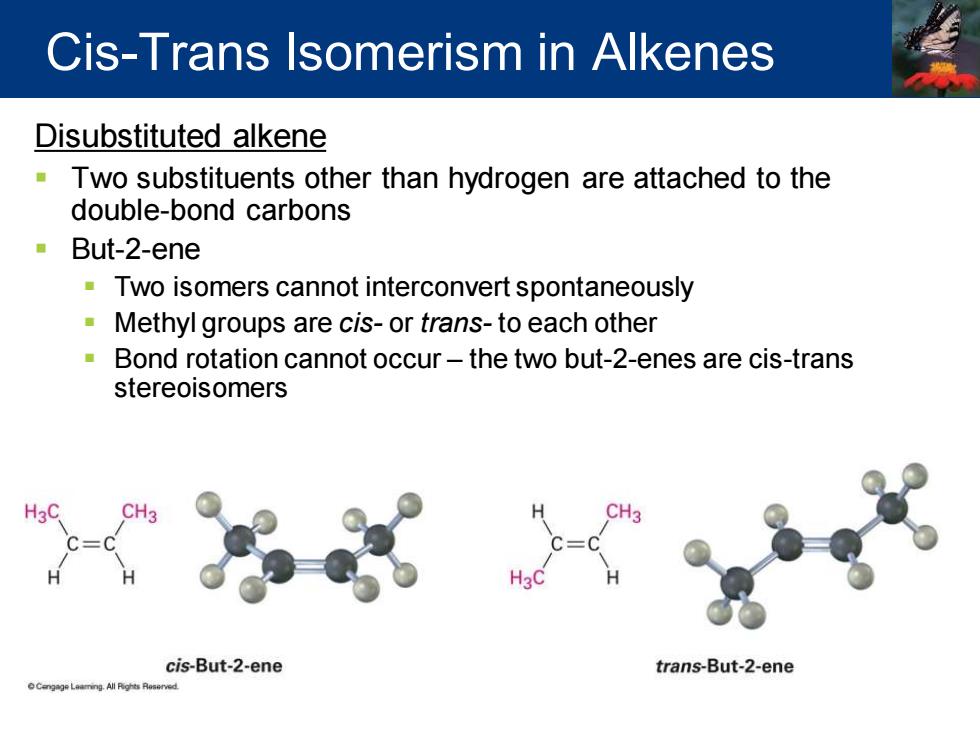
Cis-Trans Isomerism in Alkenes Disubstituted alkene Two substituents other than hydrogen are attached to the double-bond carbons But-2-ene Two isomers cannot interconvert spontaneously Methyl groups are cis-or trans-to each other Bond rotation cannot occur-the two but-2-enes are cis-trans stereoisomers cis-But-2-ene trans-But-2-ene
Disubstituted alkene ▪ Two substituents other than hydrogen are attached to the double-bond carbons ▪ But-2-ene ▪ Two isomers cannot interconvert spontaneously ▪ Methyl groups are cis- or trans- to each other ▪ Bond rotation cannot occur – the two but-2-enes are cis-trans stereoisomers Cis-Trans Isomerism in Alkenes