
Bromochlorofluoromethane is chiral Br 11111111111111111117
Br Cl H F Bromochlorofluoromethane is chiral H Cl Br F
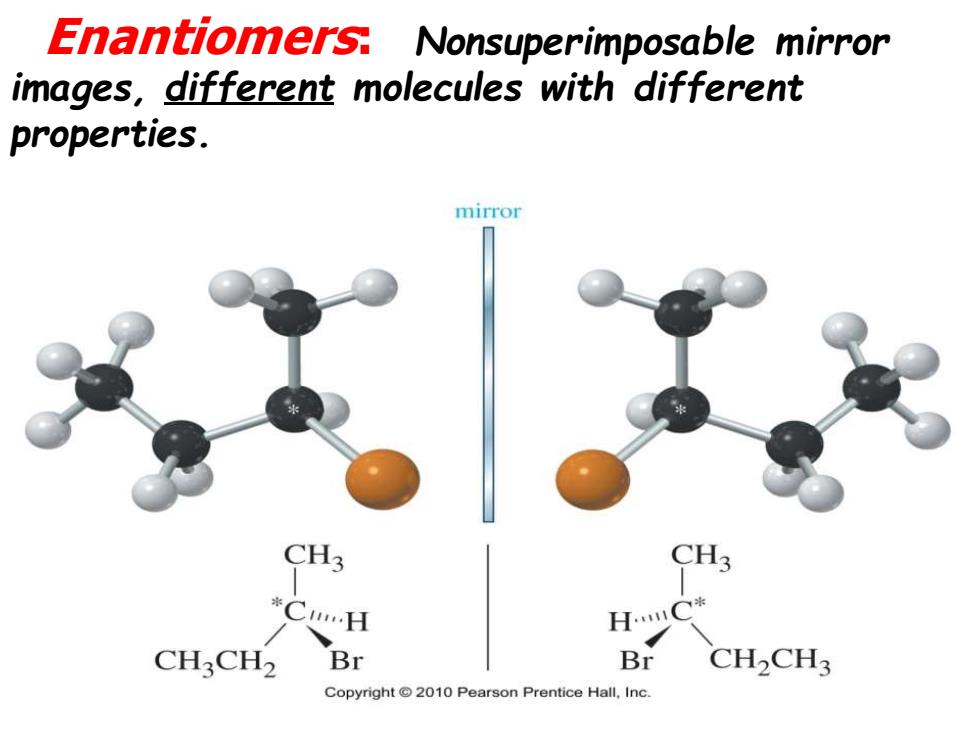
Enantiomers:Nonsuperimposable mirror images,different molecules with different properties. mirror CH3 *C…H H.C* CHCH2 Br Br CH2CH3 Copyright2010 Pearson Prentice Hall,Inc
Enantiomers: Nonsuperimposable mirror images, different molecules with different properties
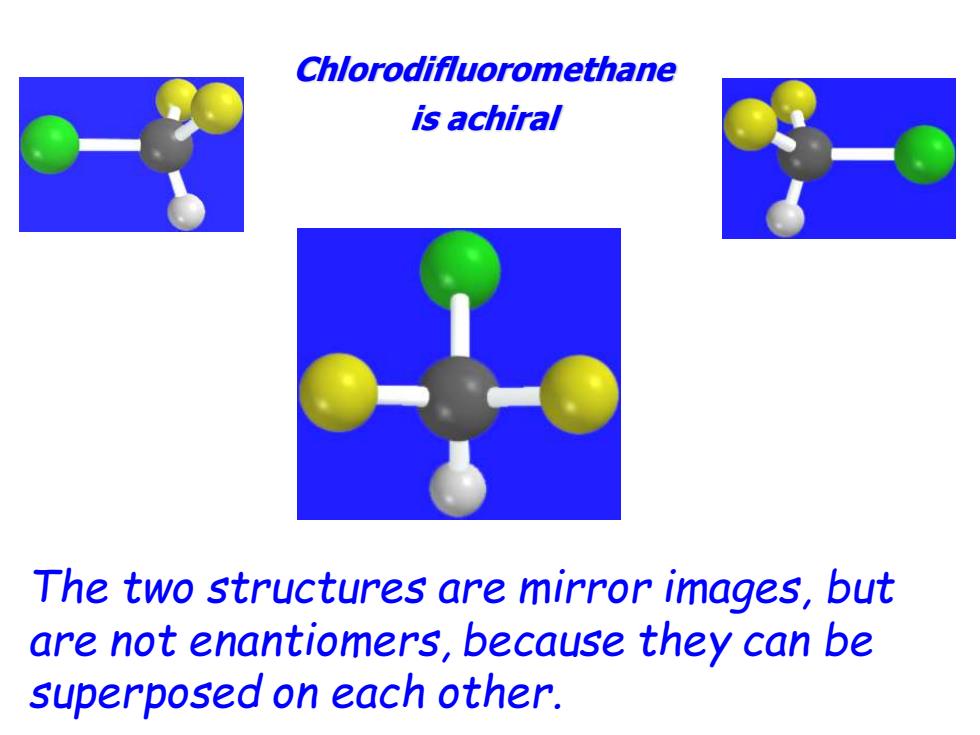
Chlorodifluoromethane is achiral The two structures are mirror images,but are not enantiomers,because they can be superposed on each other
Chlorodifluoromethane is achiral The two structures are mirror images, but are not enantiomers, because they can be superposed on each other
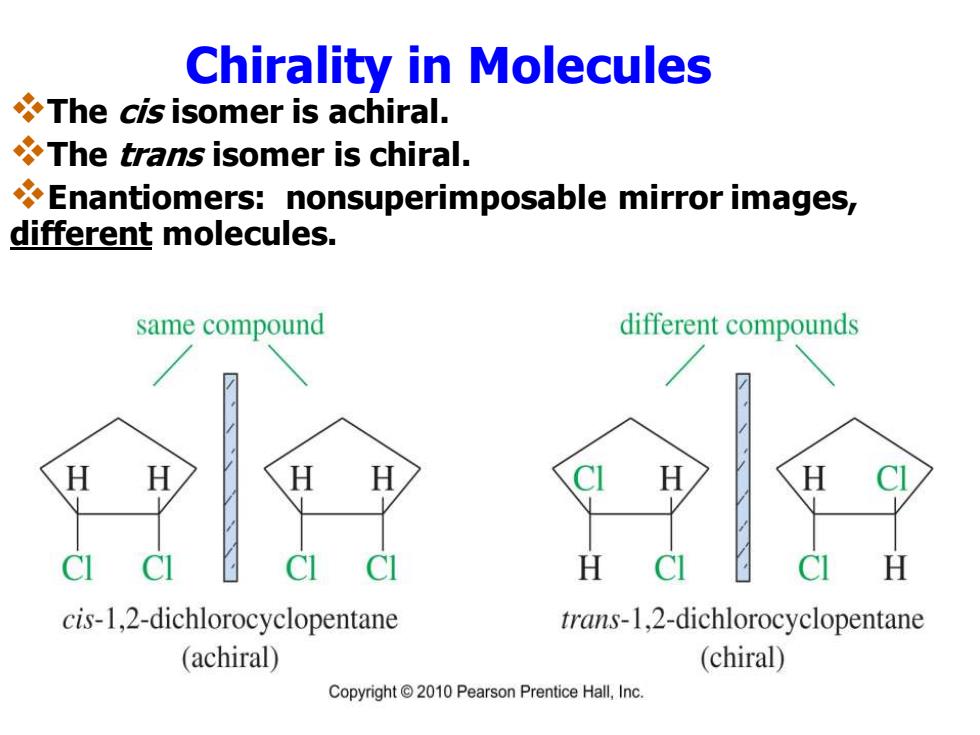
Chirality in Molecules The cis isomer is achiral. The trans isomer is chiral. Enantiomers:nonsuperimposable mirror images, different molecules. same compound different compounds H cis-1,2-dichlorocyclopentane trans-1,2-dichlorocyclopentane (achiral) (chiral) Copyright2010 Pearson Prentice Hall,Inc
Chirality in Molecules ❖The cis isomer is achiral. ❖The trans isomer is chiral. ❖Enantiomers: nonsuperimposable mirror images, different molecules
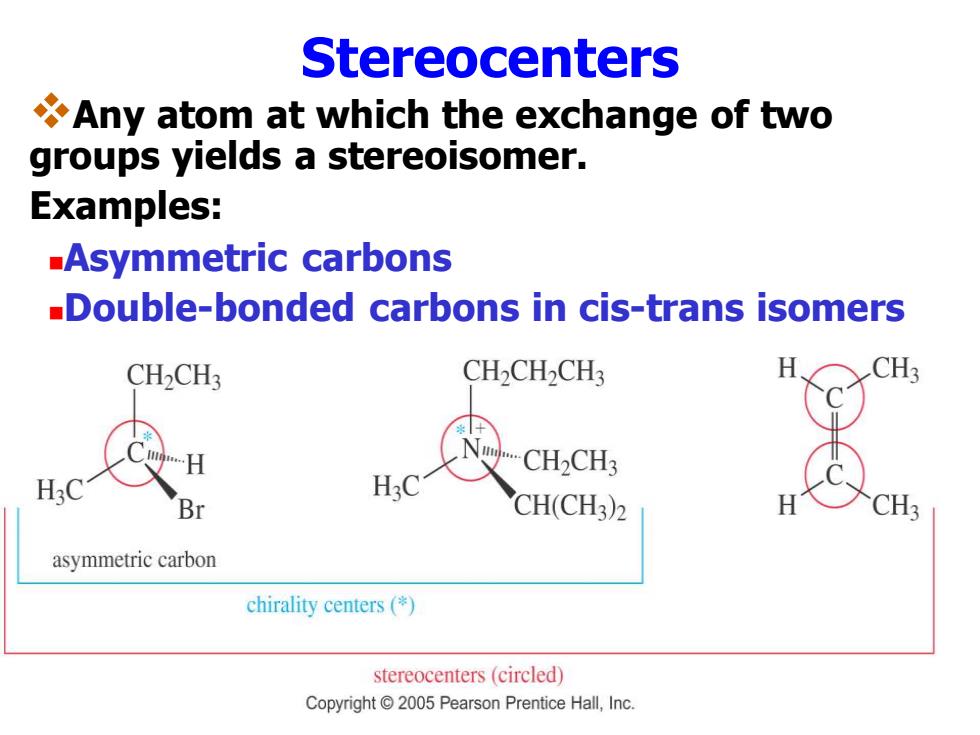
Stereocenters Any atom at which the exchange of two groups yields a stereoisomer. Examples: -Asymmetric carbons Double-bonded carbons in cis-trans isomers CH-CH3 CH>CH>CH3 CH3 CH-CH3 H:C Br CH(CH3)2 CH3 asymmetric carbon chirality centers(*) stereocenters(circled) Copyright 2005 Pearson Prentice Hall,Inc
Stereocenters ❖Any atom at which the exchange of two groups yields a stereoisomer. Examples: ◼Asymmetric carbons ◼Double-bonded carbons in cis-trans isomers