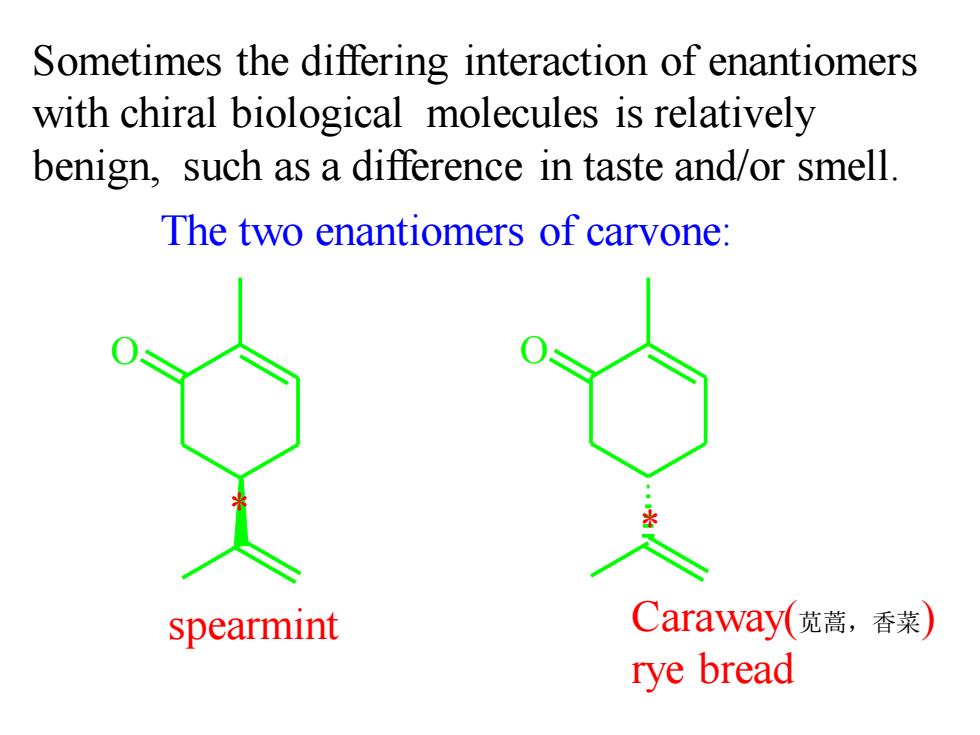
Sometimes the differing interaction of enantiomers with chiral biological molecules is relatively benign,such as a difference in taste and/or smell. The two enantiomers of carvone: spearmint Caraway(苋蒿,香菜 rye bread
O O Sometimes the differing interaction of enantiomers with chiral biological molecules is relatively benign, such as a difference in taste and/or smell. The two enantiomers of carvone: spearmint Caraway(苋蒿,香菜) rye bread * *
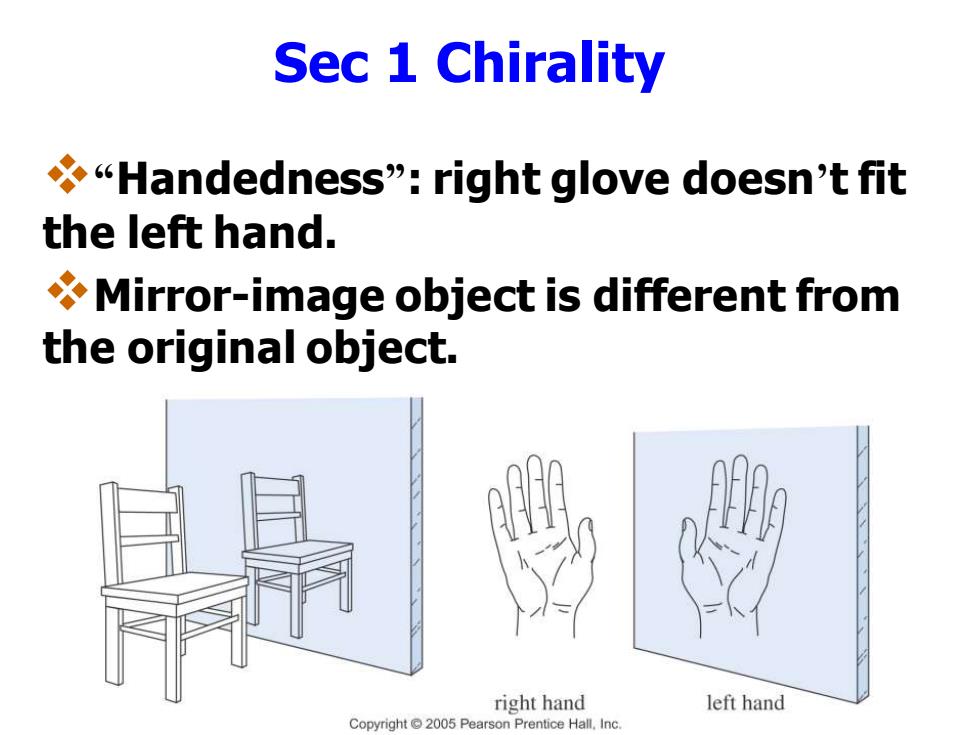
Sec 1 Chirality "Handedness":right glove doesn't fit the left hand. Mirror-image object is different from the original object. right hand left hand Copyright2005 Pearson Prentice Hall.Inc
Sec 1 Chirality ❖“Handedness”: right glove doesn’t fit the left hand. ❖Mirror-image object is different from the original object
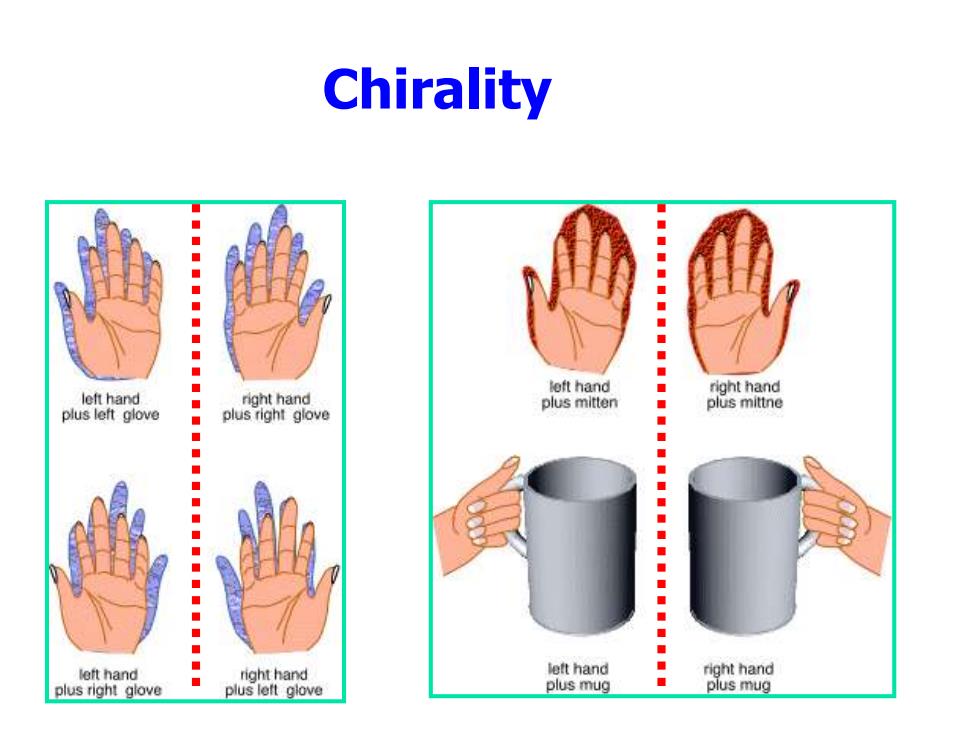
Chirality ● ● ◆ left hand right hand left hand plus left glove ◆ right hand plus mitten ◆ plus mittne plus right glove ●】 ◆ ● left hand right hand left hand right hand plus right glove plus left glove plus mug plus mug
Chirality

Chirality A molecule is chiral if its two mirror image forms are not superposable upon one another. A molecule is achiral if its two mirror image forms are superposable
Chirality ❖A molecule is chiral if its two mirror image forms are not superposable upon one another. ❖A molecule is achiral if its two mirror image forms are superposable
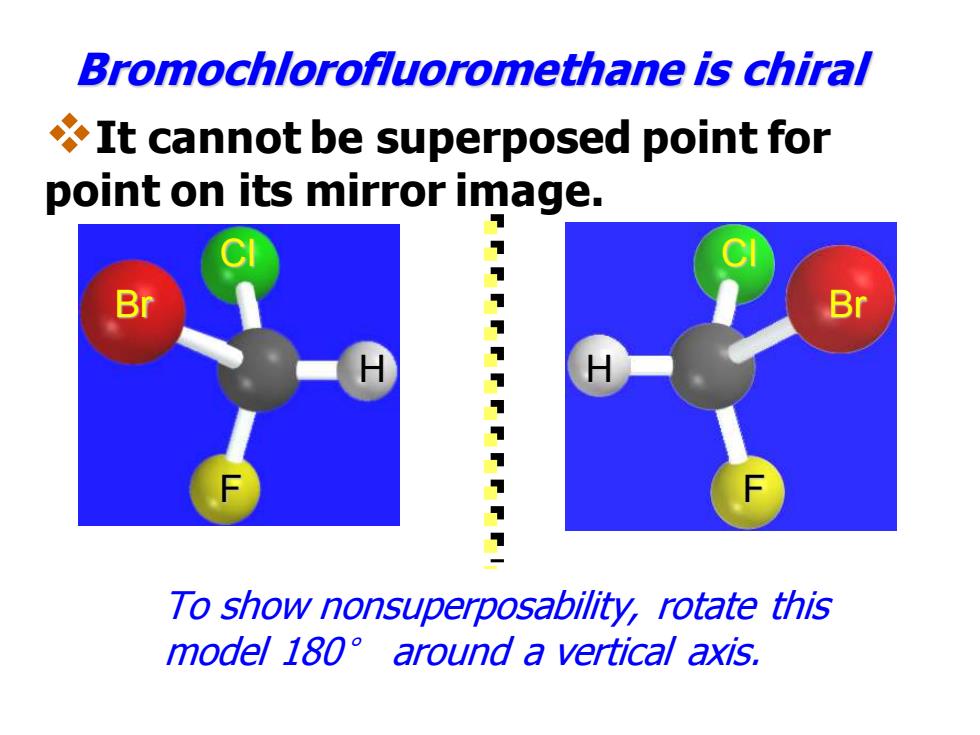
Bromochlorofluoromethane is chiral It cannot be superposed point for point on its mirror image. Br Br 11111 1 To show nonsuperposability,rotate this model 180 around a vertical axis
Br Cl H F Bromochlorofluoromethane is chiral H Cl Br F To show nonsuperposability, rotate this model 180° around a vertical axis. ❖It cannot be superposed point for point on its mirror image