
Second-order transition S.J.T.U. Phase Transformation and Applications First derivatives of G with respect to T and p are continuous and the second derivatives of G with respect to T and P are discontinuous SA=SB S=S(T,p) dS dT+ dp T dS= d7- C。=T T dp n SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 8 Chemical equilibrium
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 8 Chemical equilibrium Second-order transition First derivatives of G with respect to T and p are continuous and the second derivatives of G with respect to T and P are discontinuous S A = S B S = S(T, p) dp p S dT T S dS p T + = dp T V dT T C d S p p = − p p T S C T =

Second-order transition(2) S.J.T.U. Phase Transformation and Applications ds=dt-ar T dp ds,=dsB dsas。-0-CsC≥rm-eas-ra,p Teq VA=VR=V ACp The thermal expansion dTeg VTe△a coefficient does change! dp △ dTeg △B SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 8 Chemical equilibrium
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 8 Chemical equilibrium Second-order transition (2) dp T V dT T C d S p p = − dS A = dS B ( ) e q B B A A e q e q p B p A A B dT V V dp T C C dS dS − − − − = = , , 0 V A =V B =V = e q p e q e q VT C dT dp The thermal expansion coefficient does change! = e q e q dT dp
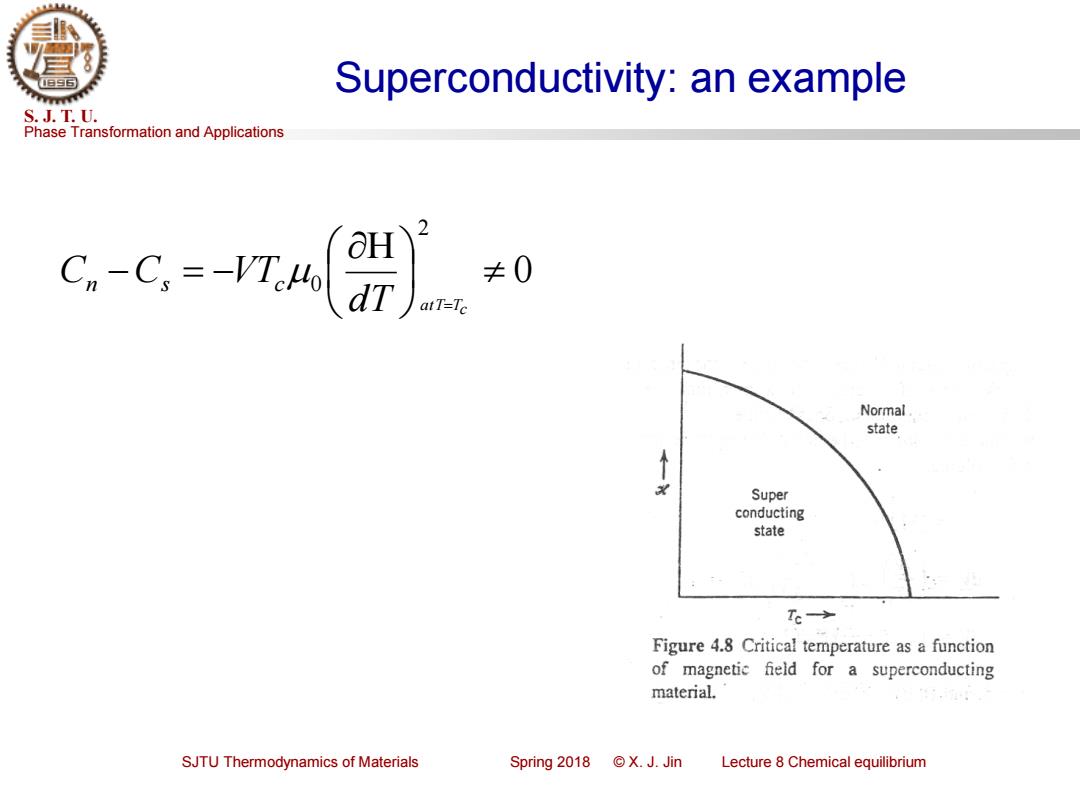
Superconductivity:an example S.J.T.U. Phase Transformation and Applications 2 C,-C.=-VTMh dr ≠0 atT-Tc Normal state Super conducting state Tc→ Figure 4.8 Critical temperature as a function of magnetie field for a superconducting material. SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 8 Chemical equilibrium
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 8 Chemical equilibrium Superconductivity: an example 0 2 0 − = − d T atT=Tc Cn Cs VTc
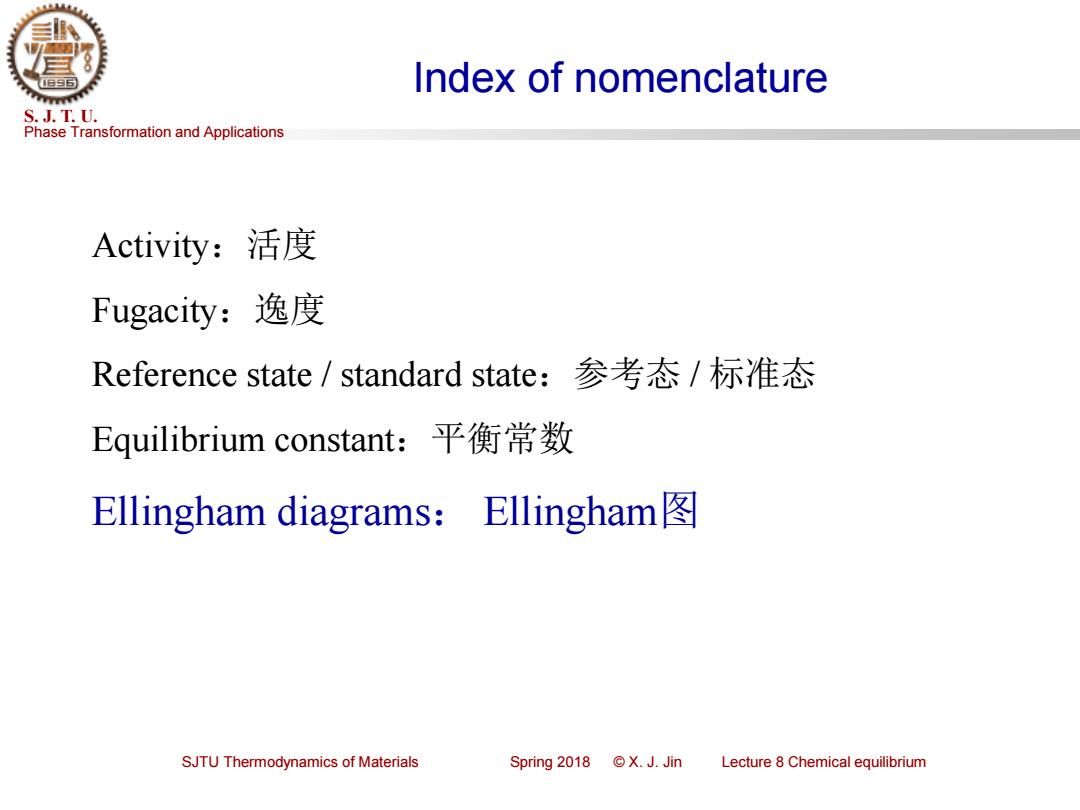
Index of nomenclature S.J.T.U. Phase Transformation and Applications Activity:活度 Fugacity:逸度 Reference state/standard state:参考态/标准态 Equilibrium constant:平衡常数 Ellingham diagrams:Ellingham SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 8 Chemical equilibrium
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 8 Chemical equilibrium Index of nomenclature Activity:活度 Fugacity:逸度 Reference state / standard state:参考态 / 标准态 Equilibrium constant:平衡常数 Ellingham diagrams: Ellingham图
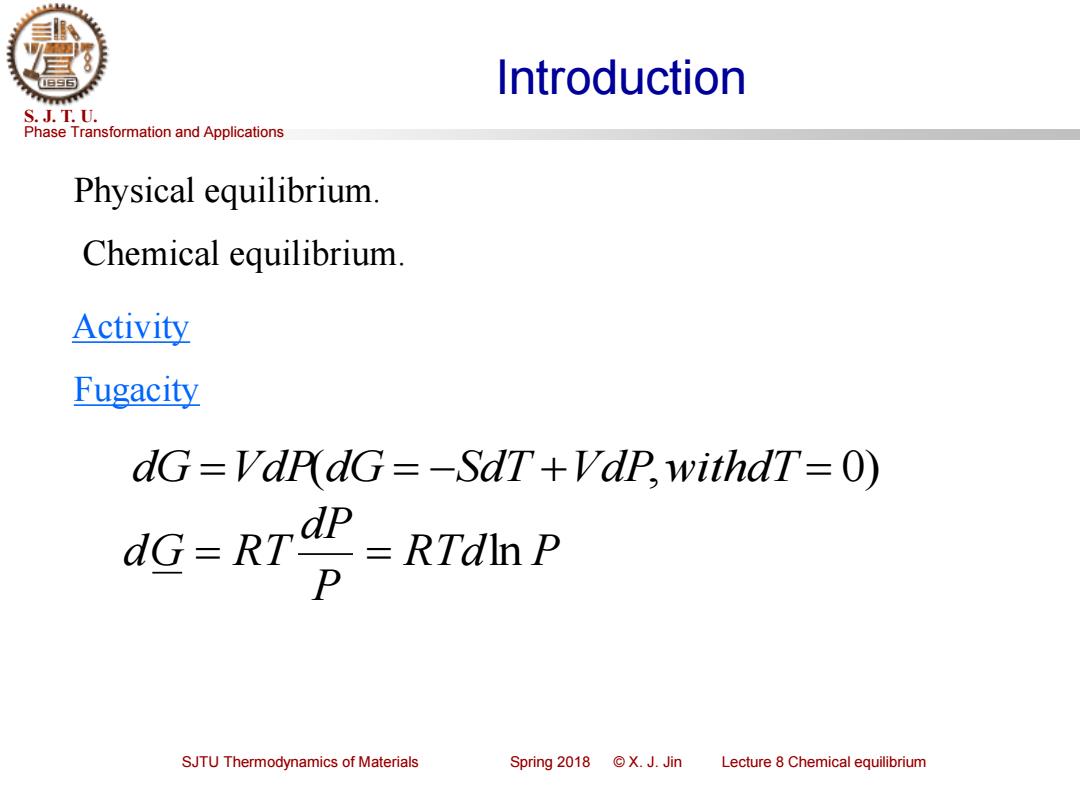
Introduction S.J.T.U. Phase Transformation and Applications Physical equilibrium. Chemical equilibrium. Activity Fugacity dG=VdrdG=-SdT+Vdp,withdT=O) dG=RTP RTdln P P SJTU Thermodynamics of Materials Spring2018©X.J.Jin Lecture 8 Chemical equilibrium
Phase Transformation and Applications S. J. T. U. SJTU Thermodynamics of Materials Spring 2018 © X. J. Jin Lecture 8 Chemical equilibrium Introduction Physical equilibrium. Chemical equilibrium. Activity Fugacity dG =VdP(dG = −SdT +VdP,withdT = 0) RTd P P dP dG = R T = ln