
Cyclopentadienyl lons -The cation has an empty p two electrons empty in p orbital orbital,4 electrons,so p orbital antiaromatic. -The anion has a nonbonding pair of electrons in a p orbital,6 e's, aromatic. four electrons six electrons cyclopentadienyl cation cyclopentadienyl anion cyclopentadienyl anion:six pi electrons,aromatic cyclopentadienyl cation:four pi electrons,antiaromatic The resonance picture gives a misleading suggestion of stability
Cyclopentadienyl Ions ▪The cation has an empty p orbital, 4 electrons, so antiaromatic. ▪The anion has a nonbonding pair of electrons in a p orbital, 6 e- ’s, aromatic

Which of the following is an aromatic Non-aromatic Aromatic There is an sp3 carbon in All carbons are sp3 the ring,delocalization will hybridized and it obeys not be complete. Huckel's rule
Which of the following is an aromatic compound? Non-aromatic Aromatic There is an sp3 carbon in the ring, delocalization will not be complete. All carbons are sp3 hybridized and it obeys Huckel’s rule

Cycloheptatrienyl Cation Planar... 6元electrons, therefore aromatic H OH H H (pH<3) H H+,H,O H H H H Tropylium lon tropylium ion,six pi electrons Copyright 2010 Pearson Prentice Hall.Inc
Tropylium Ion
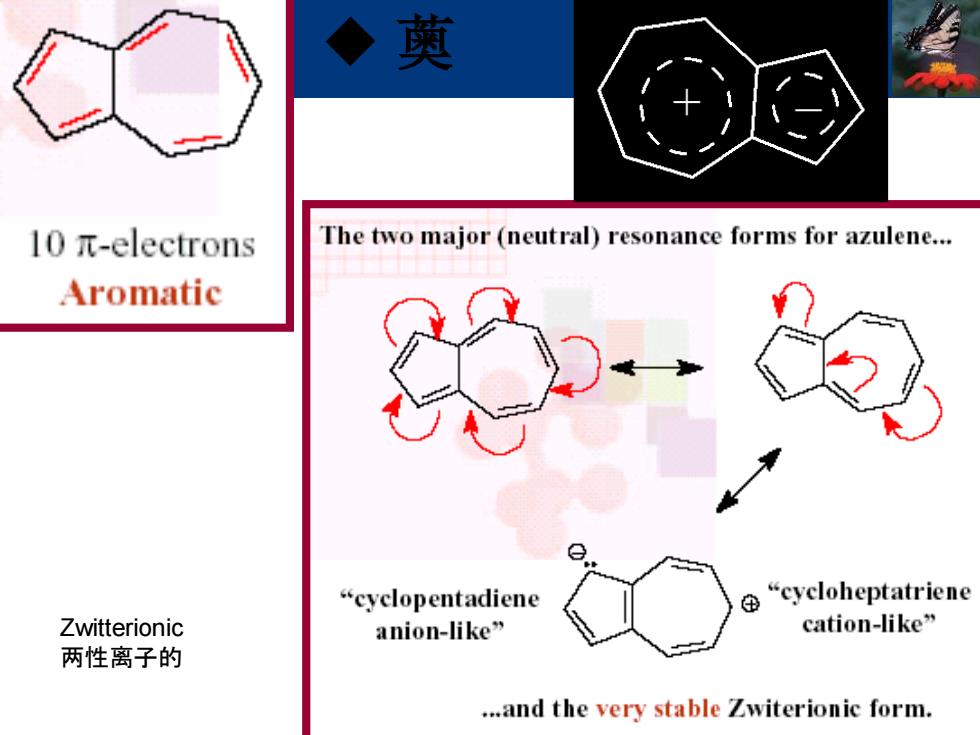
奠 l0元-electrons The two major(neutral)resonance forms for azulene... Aromatic “cyclopentadiene ⊕ “cycloheptatriene Zwitterionic anion-like” cation-like” 两性离子的 ...and the very stable Zwiterionic form
◆ 薁 Zwitterionic 两性离子的

8 薁 5 8 >350℃ 异构化 5 3
薁