
Properties of Ar compounds -Many aromatic compounds have a characteristic aroma and burn with a smoky flame. -They are nonpolar,hydrophobic molecules which dissolve in organic solvents rather than water. -Aromatic molecules can interact by van der Waals interactions or with a cation through an induced dipole interaction. -Electrophilic substitution is the most common type of reaction.However,reduction is also possible. b) M van der Waals interactions Induced dipole interaction Fig.1.Intermolecular bonding involving aromatic rings
Properties of Ar compounds ▪Many aromatic compounds have a characteristic aroma and burn with a smoky flame. ▪They are nonpolar, hydrophobic molecules which dissolve in organic solvents rather than water. ▪Aromatic molecules can interact by van der Waals interactions or with a cation through an induced dipole interaction. ▪Electrophilic substitution is the most common type of reaction. However, reduction is also possible

Sec 2 Aromaticity and Reactivity Reactivity,反应性 ■不易加成(苯环骨架不断开) ·发生取代(亲电取代E.S) ·侧链反应 Aromaticity芳香性化合物特点 ■大的不饱和度(2≥4)2=? ·(环状)平面分子 ·易进行亲电取代反应 芳香性
Sec 2 Aromaticity and Reactivity ▪Reactivity反应性 ▪ 不易加成(苯环骨架不断开) ▪ 发生取代(亲电取代 E.S) ▪ 侧链反应 ▪Aromaticity芳香性化合物特点 ▪ 大的不饱和度(≧4) =? ▪ (环状)平面分子 ▪ 易进行亲电取代反应 芳香性
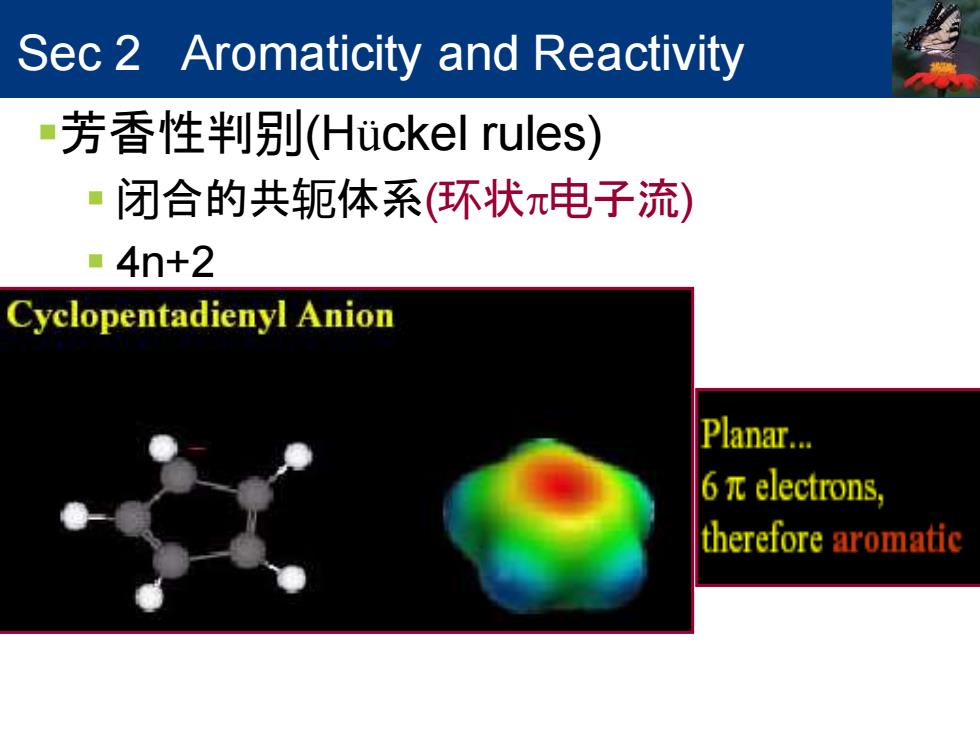
Sec 2 Aromaticity and Reactivity ■芳香性判别(Huckel rules) ■闭合的共轭体系(环状π电子流) ·4n+2 Cyclopentadienyl Anion Planar... 6πelectrons, therefore aromatic
Sec 2 Aromaticity and Reactivity ▪芳香性判别(Hückel rules) ▪ 闭合的共轭体系(环状电子流) ▪ 4n+2
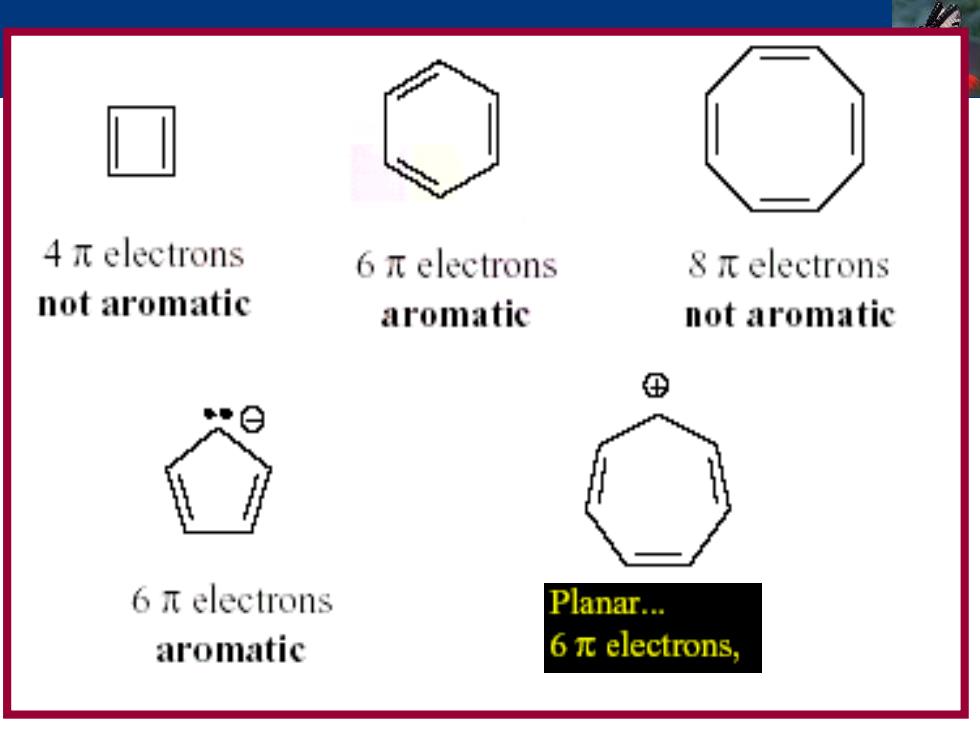
4πelectrons 6πelectrons 8πelectrons not aromatic aromatic not aromatic ⊕ 6πelectrons Planar... aromatic 6πelectrons
Huickel's Rule Once the aromatic criteria is met,Huckel's rule applies. If the number of pi electrons is (4N 2)the compound is aromatic (where N is an integer) If the number of pi electrons is(4N)the compound is antiaromatic
Hückel’s Rule ▪ Once the aromatic criteria is met, Huckel’s rule applies. ▪ If the number of pi electrons is (4N + 2) the compound is aromatic (where N is an integer) ▪ If the number of pi electrons is (4N) the compound is antiaromatic