
16.1. 2 General physical properties High m.p and b.p Tungsten (W)has the highest m.p among the elemental substances ·High hardness The hardest metal:Cr High densities The most dense metal:Os Good conductor of heat and electricity,and good ductibility(延展性)
16.1.2 General physical properties • High m.p and b.p Tungsten (W) has the highest m.p among the elemental substances • High hardness The hardest metal: Cr • High densities The most dense metal: Os • Good conductor of heat and electricity, and good ductibility(延展性 )

3500 Re 3000 Ta 6th row Mo 2500 Ir melting points Hf 2000 5th row Rh Pt Lu r Ti Fe 1500 Sc Co Pd N 4th row Mn Cu 1000 Au 500 ●Zn >Cd 0 Hg IIA IIIB IVB VB VIB VIB W IB IIB A plot showing the trend of melting point of the transition metals
A plot showing the trend of melting point of the transition metals melting points 6th row 5th row 4th row
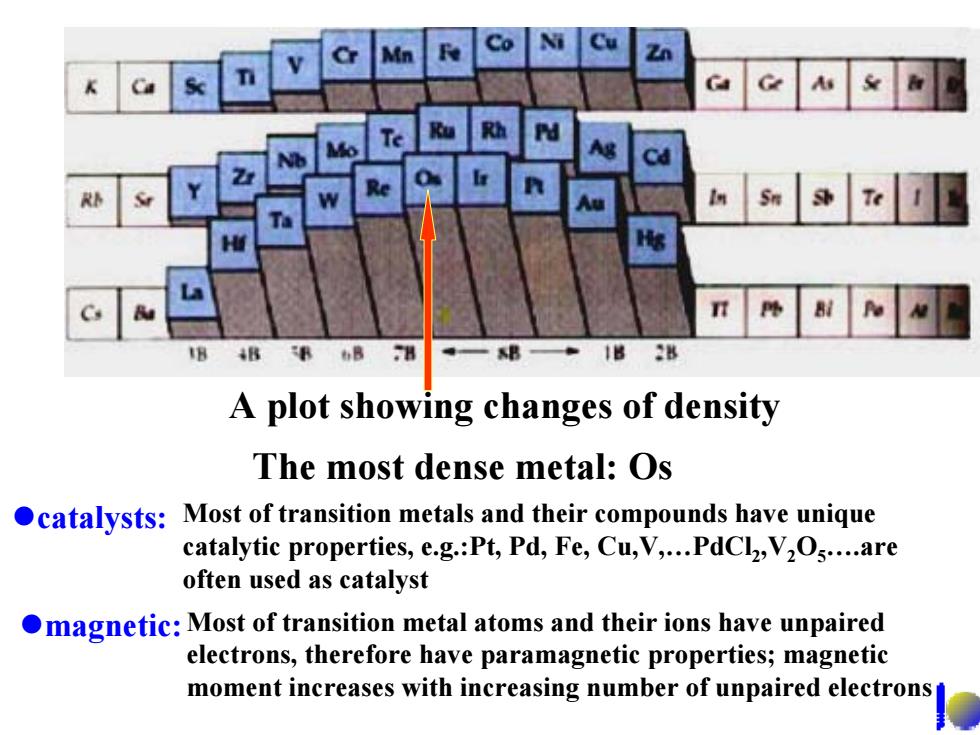
4B B 6B 28 A plot showing changes of density The most dense metal:Os catalysts:Most of transition metals and their compounds have unique catalytic properties,e.g.:Pt,Pd,Fe,Cu,V,...PdCl,V2Os....are often used as catalyst magnetic:Most of transition metal atoms and their ions have unpaired electrons,therefore have paramagnetic properties;magnetic moment increases with increasing number of unpaired electrons
A plot showing changes of density The most dense metal: Os zcatalysts: Most of transition metals and their compounds have unique catalytic properties, e.g.:Pt, Pd, Fe, Cu,V,…PdCl 2,V 2 O 5….are often used as catalyst zmagnetic:Most of transition metal atoms and their ions have unpaired electrons, therefore have paramagnetic properties; magnetic moment increases with increasing number of unpaired electrons
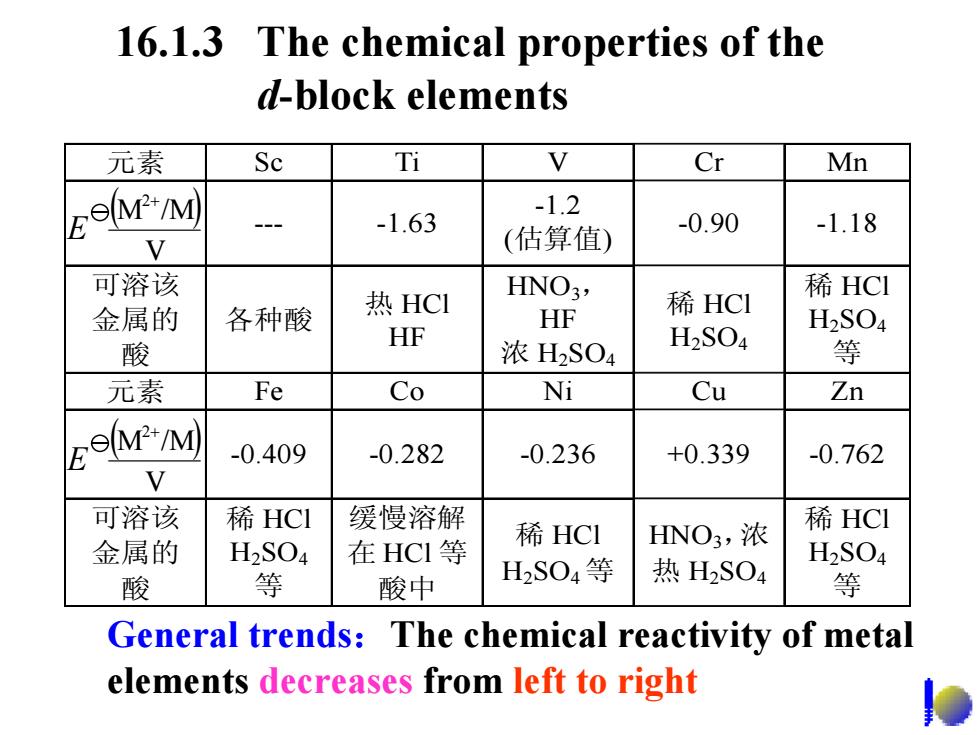
16.1.3 The chemical properties of the d-block elements 元素 Sc Cr Mn lM"M) -1.2 -1.63 -0.90 -1.18 V (估算值) 可溶该 热HCI HNO3, 稀HCI 稀HCI 金属的 各种酸 HF H2SO4 HF 酸 浓H2SO4 H2SO4 等 元素 Fe Co Ni Cu Zn lem"M) -0.409 -0.282 -0.236 +0.339 -0.762 V 可溶该 稀HCI 缓慢溶解 稀HCl HNO3,浓 稀HCI 金属的 H2SO4 在HCI等 H2S04等 热H2SO4 H2SO4 酸 等 酸中 等 General trends:The chemical reactivity of metal elements decreases from left to right
16.1.3 The chemical properties of the d-block elements General trends :The chemical reactivity of metal elements decreases from left to right 元素 Sc Ti V Cr Mn --- -1.63 -1.2 (估算值 ) -0.90 -1.18 可溶该 金属的 酸 各种酸 热 HCl HF HNO 3, HF 浓 H 2SO 4 稀 HCl H 2SO 4 稀 HCl H 2SO 4 等 元素 Fe Co Ni Cu Zn -0.409 -0.282 -0.236 +0.339 -0.762 可溶该 金属的 酸 稀 HCl H 2SO 4 等 缓慢溶解 在 HCl 等 酸中 稀 HCl H 2SO 4 等 HNO 3,浓 热 H 2SO 4 稀 HCl H 2SO 4 等 ( ) V M /M 2 + E ( ) V M /M 2 + E
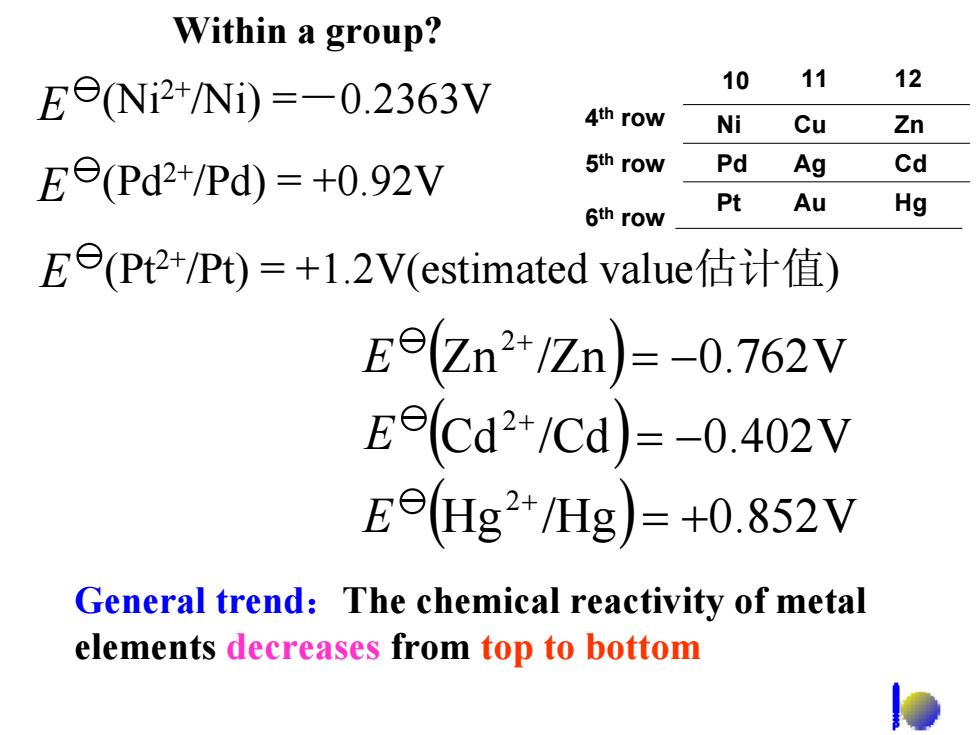
Vithin a group? EeNi2+/Ni))=-0.2363V 10 11 12 4th row Ni Cu Zn Ee(Pd2+/Pd)=+0.92V 5th row Pd Ag Cd 6th row Pt Au Hg E(Pt2+/Pt)=+l.2V(estimated value估计值) Ee(Zn2+/Zn)=-0.762V Ee(Cd2+/Cd)=-0.402V E©(Hg2+/Hg)=+0.852V General trend:The chemical reactivity of metal elements decreases from top to bottom
General trend:The chemical reactivity of metal elements decreases from top to bottom ( ) ( ) ( ) Hg /Hg 0.852V Cd /Cd 0.402V Zn /Zn 0.762V 2 2 2 = + = − = − + + + E E E E (Ni2+/Ni) =-0.2363V E (Pd2+/Pd) = +0.92V E (Pt2+/Pt) = +1.2V(estimated value估计值) Within a group? 4th row 5th row 6th row Ni Pd Pt Cu Ag Au Zn Cd Hg 10 11 12