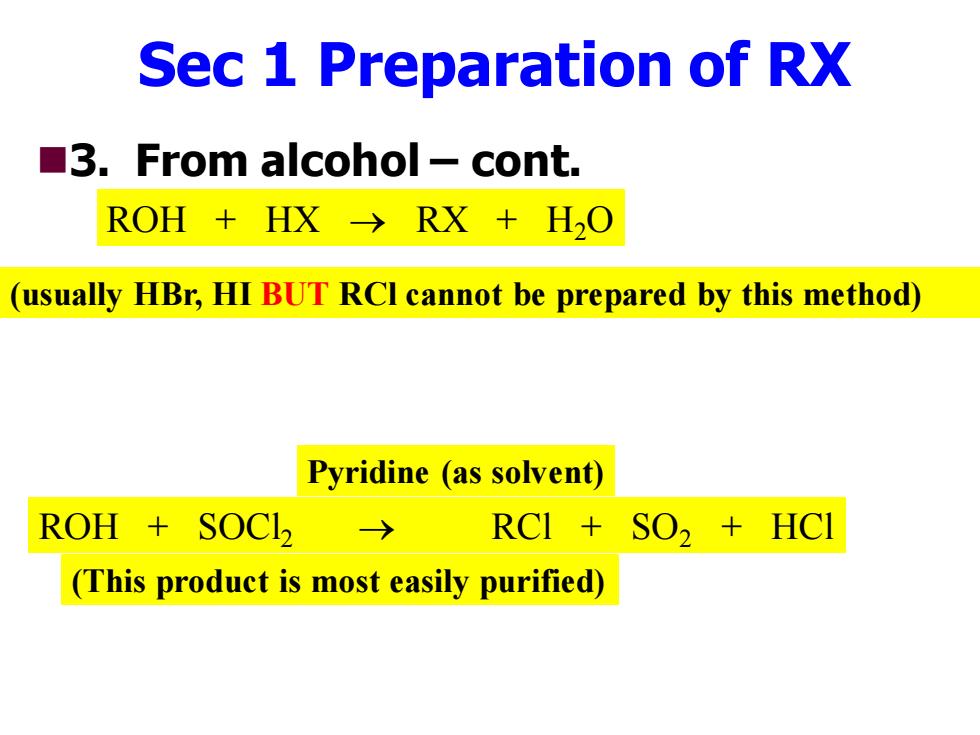
Sec 1 Preparation of RX 3.From alcohol-cont. ROH+HX→RX+HO (usually HBr,HI BUT RCI cannot be prepared by this method) Pyridine (as solvent) ROH SOCI 〉 RCI SO,HCI (This product is most easily purified)
Sec 1 Preparation of RX ◼3. From alcohol – cont. ROH + HX → RX + H2O (usually HBr, HI BUT RCl cannot be prepared by this method) ROH + SOCl2 → RCl + SO2 + HCl Pyridine (as solvent) (This product is most easily purified)
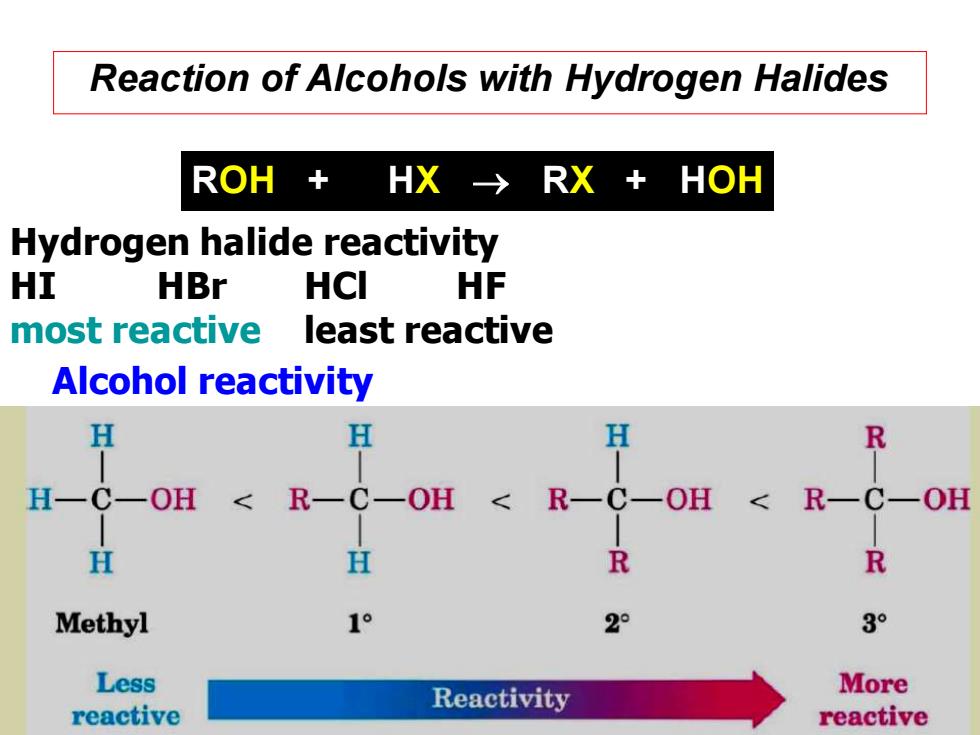
Reaction of Alcohols with Hydrogen Halides ROH+ HX-→RX+HOH Hydrogen halide reactivity HI HBr HCI HF most reactive least reactive Alcohol reactivity H H H R H一C一OH <R一C-OH R- H H R R Methyl 1° 2° 3° Less Reactivity More reactive reactive
Reaction of Alcohols with Hydrogen Halides ROH + HX → RX + HOH Hydrogen halide reactivity HI HBr HCl HF most reactive least reactive Alcohol reactivity
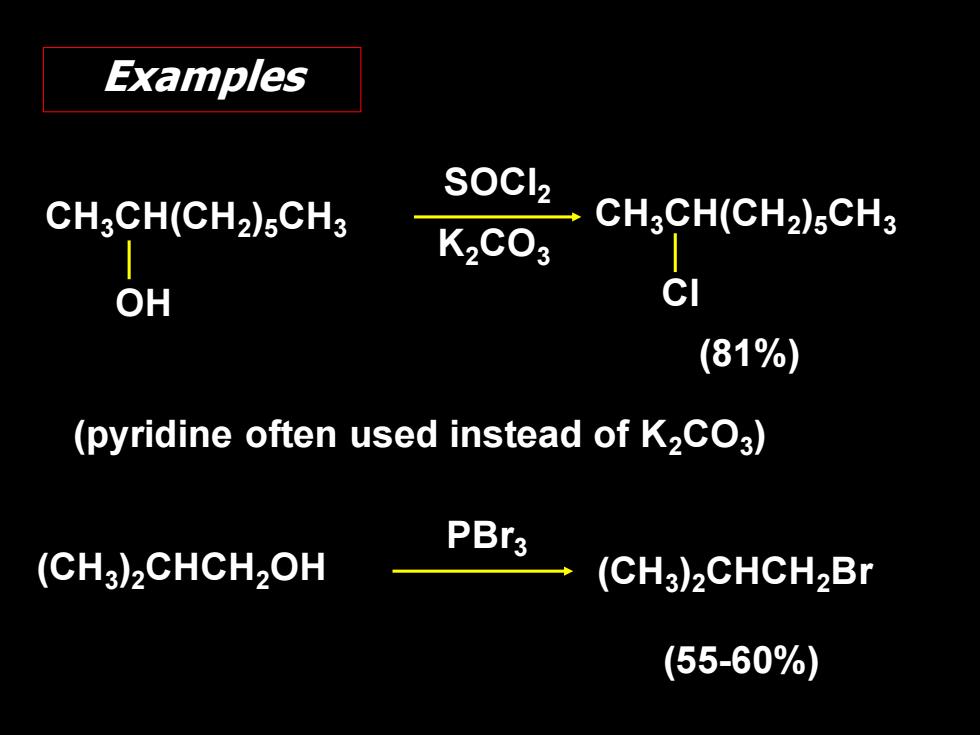
Examples CH3CH(CH2)sCH3 SOCl2 CHCH(CHa)sCH3 K2C03 OH cl (81%) (pyridine often used instead of K2CO3) PBr3 (CH3)2CHCH2OH (CH3)2CHCH2Br (55-60%)
CH3CH(CH2 )5CH3 OH SOCl2 K2CO3 CH3CH(CH2 )5CH3 Cl (81%) (pyridine often used instead of K2CO3 ) (CH3 )2CHCH2OH (55-60%) (CH3 )2CHCH2Br PBr3 Examples
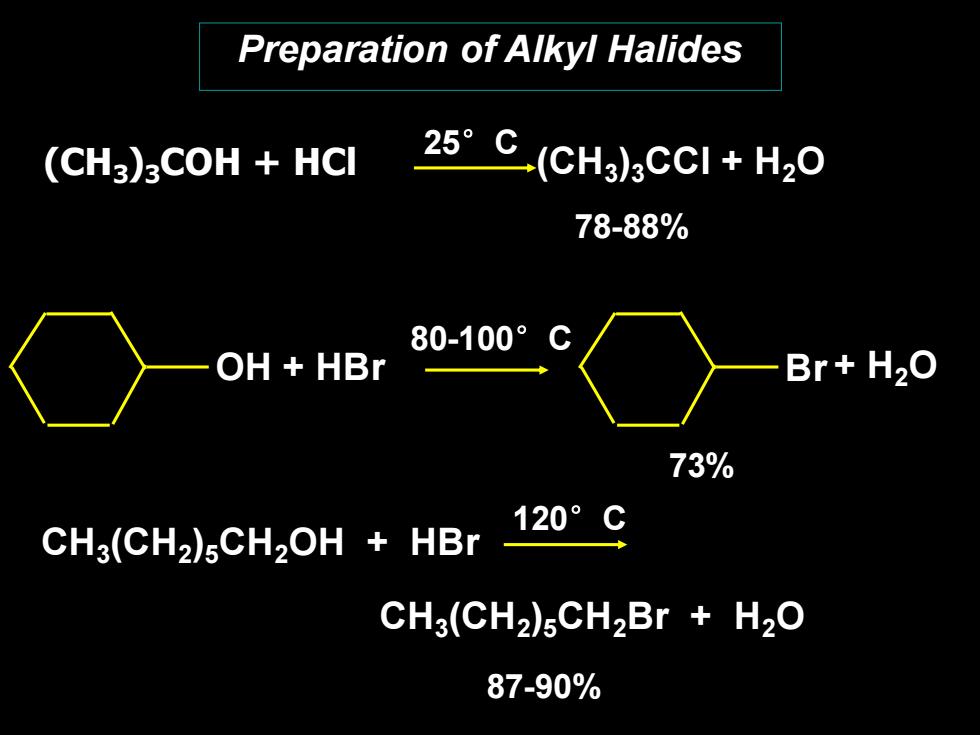
Preparation of Alkyl Halides (CH3)3COH HCI 25°C(CHg3cCI+H20 78-88% 80-100°C OH HBr Br+H2O 73% 120°C CH3(CH2)5CH2OH HBr CH3(CH2)5CH2Br H20 87-90%
Preparation of Alkyl Halides (CH3)3COH + HCl (CH3 )3CCl + H2O 78-88% + H2O 73% CH3 (CH2 )5CH2OH + HBr CH3 (CH2 )5CH2Br + H2O 87-90% 25°C 80-100°C 120°C OH + HBr Br
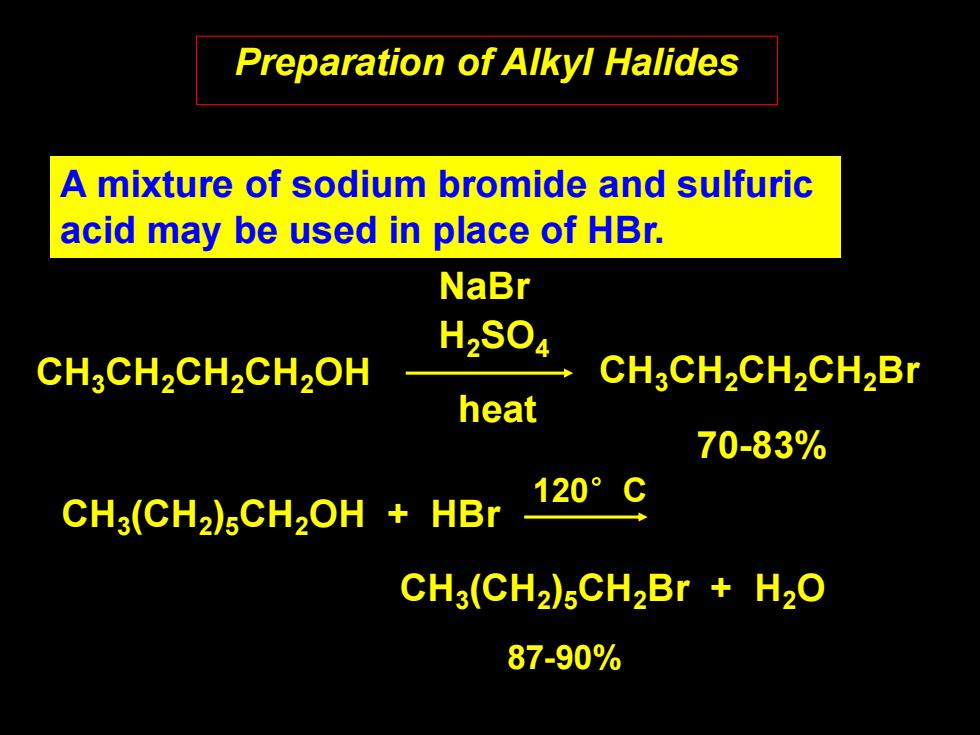
Preparation of Alkyl Halides A mixture of sodium bromide and sulfuric acid may be used in place of HBr. NaBr H2S04 CH3CH2CH2CH2OH CH3CH2CH2CH2Br heat 70-83% 120°C CH3(CH2)5CH2OH HBr CH3(CH2)5CH2Br H2O 87-90%
Preparation of Alkyl Halides CH3CH2CH2CH2OH CH3CH2CH2CH2Br NaBr H2SO4 70-83% heat A mixture of sodium bromide and sulfuric acid may be used in place of HBr. CH3 (CH2 )5CH2OH + HBr CH3 (CH2 )5CH2Br + H2O 87-90% 120°C