
Allylic Halogenation Avoid a large excess of Br2 by using N-bromosuccinimide (NBS)to generate Br2 as product HBr is formed. N-bromosuccinimide (NBS)is an allylic brominating agent. Keeps the concentration of Br2 low. EBr+HBr Br2
Allylic Halogenation ◼Avoid a large excess of Br2 by using N-bromosuccinimide (NBS) to generate Br2 as product HBr is formed. ◼N-bromosuccinimide (NBS) is an allylic brominating agent. ⚫Keeps the concentration of Br2 low. N O O Br + HBr N O O H + Br2
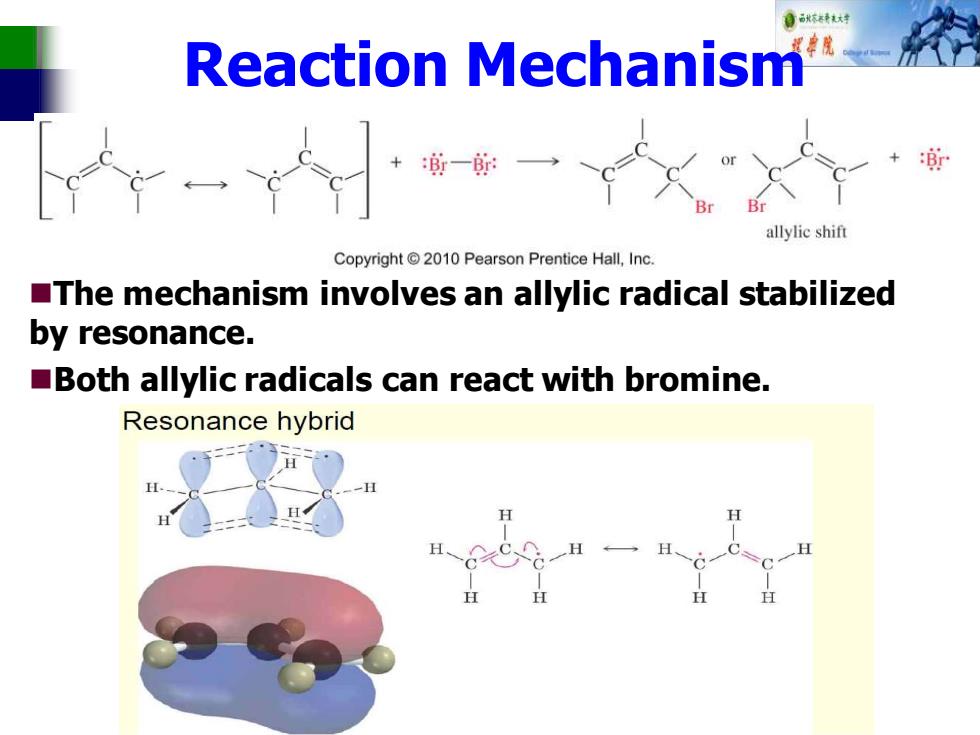
自秋特大材 Reaction Mechanism +一一 allylic shift Copyright2010 Pearson Prentice Hall,Inc. The mechanism involves an allylic radical stabilized by resonance. Both allylic radicals can react with bromine. Resonance hybrid
Reaction Mechanism ◼The mechanism involves an allylic radical stabilized by resonance. ◼Both allylic radicals can react with bromine
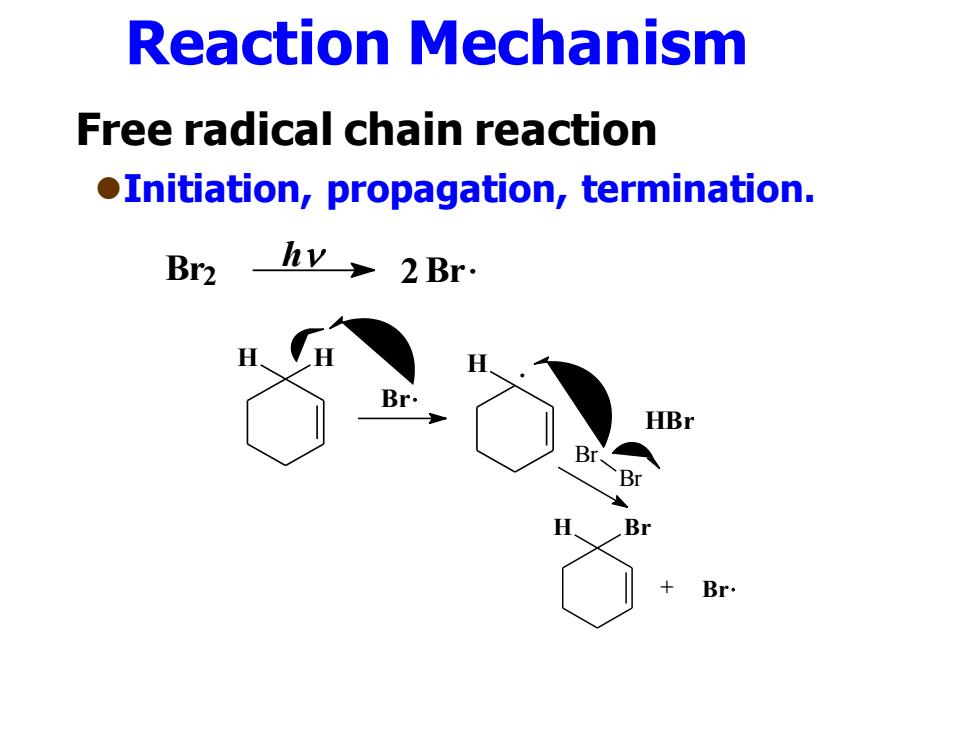
Reaction Mechanism Free radical chain reaction oInitiation,propagation,termination. Br2hY→2Br. Br. HBr Br、 Br H Br + Br
Reaction Mechanism Free radical chain reaction ⚫Initiation, propagation, termination. H H Br H + HBr Br Br H Br + Br Br2 2Br h

Sec 1 Preparation of RX ■2.Vith HX HX reflux HX is coming from Nax conc.H2SO
Sec 1 Preparation of RX ◼2. With HX C C HX reflux C C H X HX is coming from NaX + conc. H2SO4

Sec 1 Preparation of RX ■3.From alcohol ROH PCls RCI POCI HCI (phosphorus oxychloride) ROH+PCL3→RCI+HPO ROH P +Br2 →RBr+H3PO3 We rarely start with PCly PBry or PIy since they are easily hydrolysed by moisture in air.Instead,they are made in situ,using red phosphorus in the alcohol and halogen is put into the reaction flask as the reaction proceeds
Sec 1 Preparation of RX ◼3. From alcohol ◼We rarely start with PCl3 , PBr3 or PI3 , since they are easily hydrolysed by moisture in air. Instead, they are made in situ, using red phosphorus in the alcohol and halogen is put into the reaction flask as the reaction proceeds. ROH + PCl 5 RCl + POCl 3 + HCl (phosphorus oxychloride) ROH + PCl 3 RCl + H3 PO3 ROH + P + Br 2 RBr + H3 PO3