
Sec 1 PREPARATION AND PHYSICAL PROPERTIES ■PREPARATION .Halogenation(Allylic Halogenation) ●With HX ●From alcohol .Diazonium coupling (for aryl halide only) ■Use
Sec 1 PREPARATION AND PHYSICAL PROPERTIES ◼PREPARATION ⚫Halogenation(Allylic Halogenation) ⚫With HX ⚫From alcohol ⚫Diazonium coupling (for aryl halide only) ◼Use
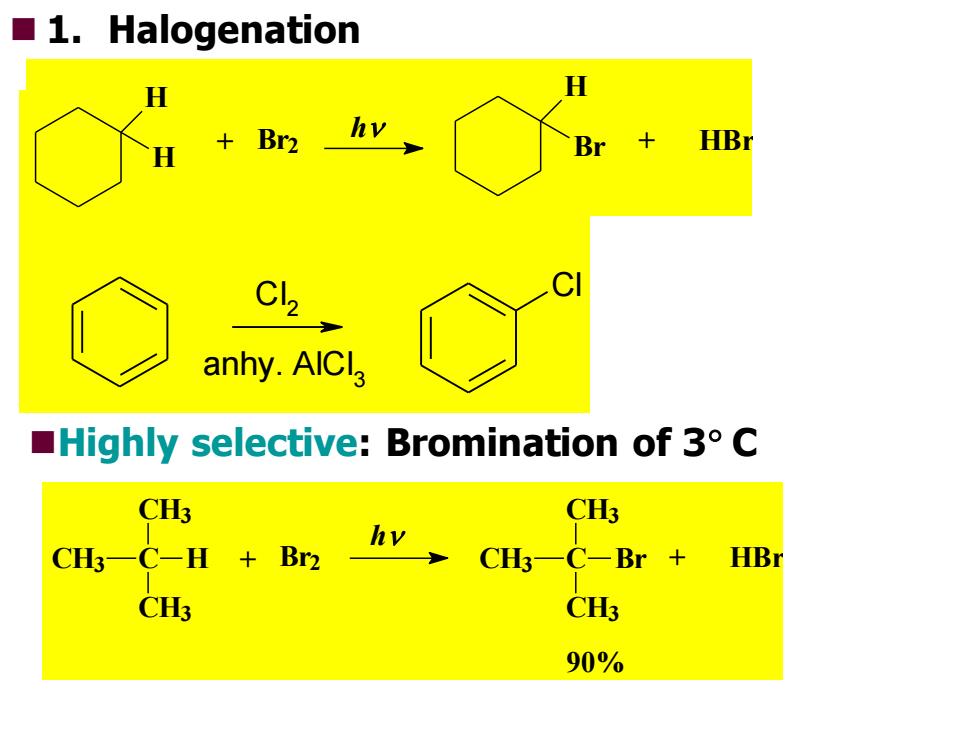
■l.Halogenation H H h +Br2 Br HB anhy.AICla Highly selective:Bromination of 3 C CH3 CH3 CH一C-H +Br2 hv CHC-Br 十 HBr CH3 CH3 90%
◼Highly selective: Bromination of 3 C 90% CH3 C + HBr CH3 CH3 Br h CH3 C + Br2 CH3 CH3 H RH + Cl 2 RCl + HCl u.v. C Cl l 2 anhy. AlCl 3 ◼ 1. Halogenation + HBr H Br h + Br2 H H

Sec 1 Preparation of RX Free radical halogenation produces mixtures,not good lab synthesis ounless:all H's are equivalent,or ohalogenation is highly selective. Free radical allylic halogenation oproduces alkyl halide with double bond on the neighboring carbon
Sec 1 Preparation of RX ◼Free radical halogenation ⚫produces mixtures, not good lab synthesis ⚫unless: all H’s are equivalent, or ⚫halogenation is highly selective. ◼Free radical allylic halogenation ⚫produces alkyl halide with double bond on the neighboring carbon

自秋转达对 Allylic Halogenation allylic positions allylic hydrogens HH H Br2 w HBr cyclohexene H 3-bromocyclohexene (80%) Copyright 2010 Pearson Prentice Hall,Inc. Allylic radical is resonance stabilized. Bromination occurs with good yield at the allylic position (sp3 C next to C=C)
Allylic Halogenation ◼Allylic radical is resonance stabilized. ◼Bromination occurs with good yield at the allylic position (sp3 C next to C=C)
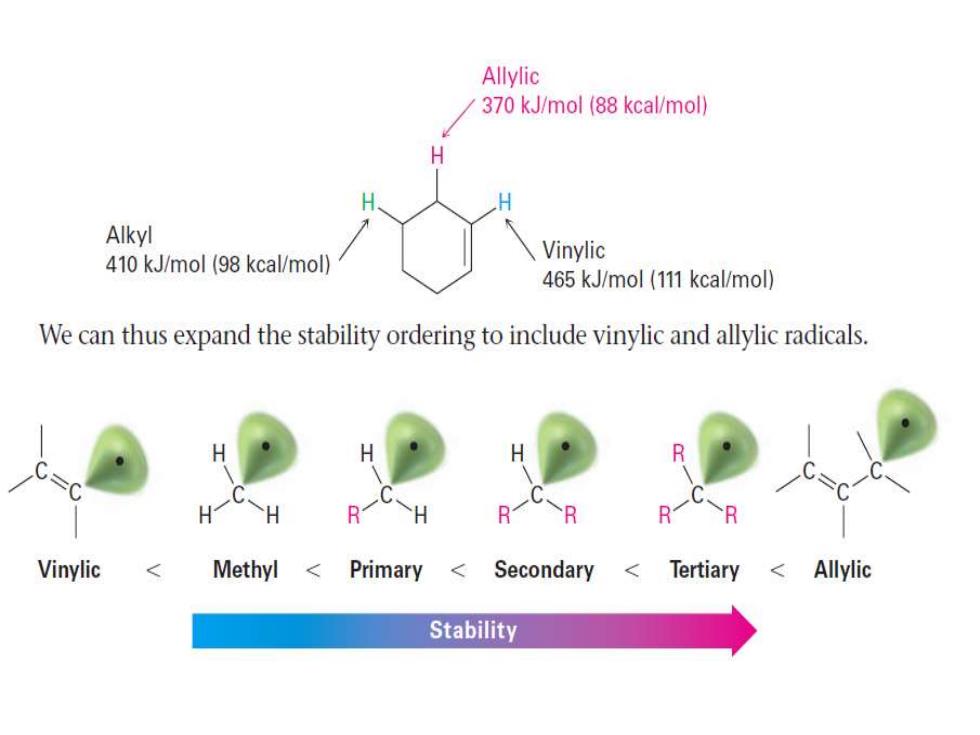
Allylic 370 kJ/mol (88 kcal/mol) H Alkyl 410 kJ/mol (98 kcal/mol) Vinylic 465 kJ/mol (111 kcal/mol) We can thus expand the stability ordering to include vinylic and allylic radicals. H H R R H R1 R Vinylic Methyl Primary < Secondary Tertiary <Allylic Stability