
Unique property of carbon Solid carbon structures Graphite Diamond Graphene Buckyball” Soot 煤烟,烟灰 Harcourt Brace Company items and derived items copyright 1998 by Harcourt Brace Company
Unique property of carbon 煤烟, 烟灰 Graphene
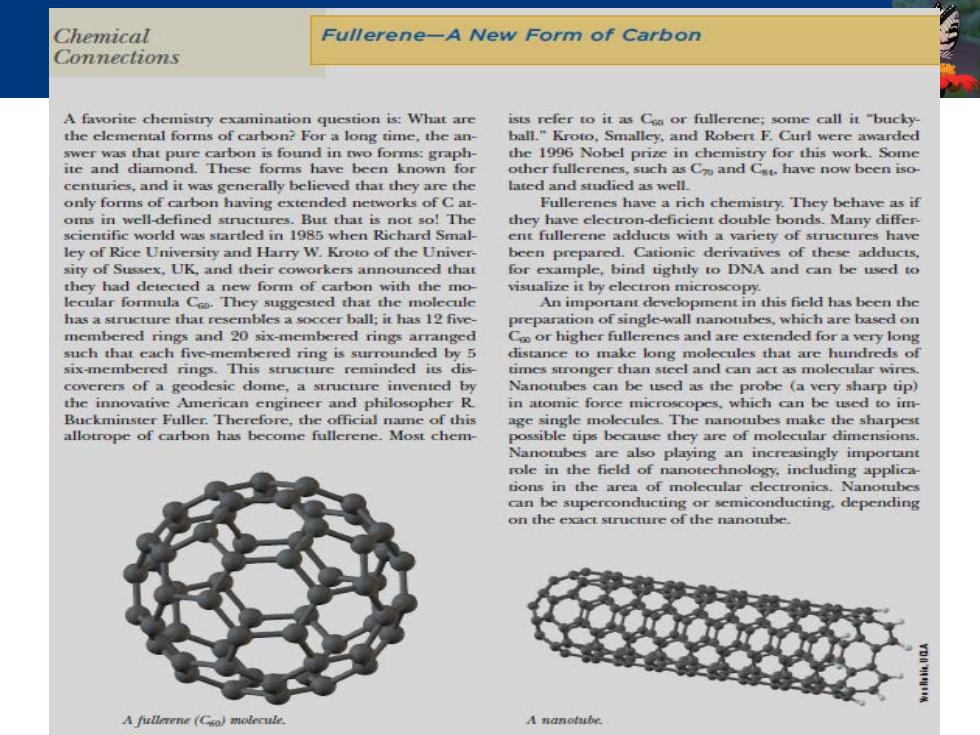
Chemical Fullerene-A New Form of Carbon Connections A favorite chemistry examination question is:What are ists refer to it as Ca or fullerene;some call it "bucky the elemental forms of carbon?For a long time,the an- ball."Kroto,Smalley,and Robert F.Curl were awarded swer was that pure carbon is found in two forms:graph- the 1996 Nobel prize in chemistry for this work.Some ite and diamond.These forms have been known for other fullerenes,such as Ca have now been iso centurics,and it was generally believed that they are the lated and studied as well. only forms of carbon having extended networks of C at- Fullerenes have a rich chemistry.They behave as if oms in well-defined structures.But that is not so!The they have clectron-deficient double bonds.Many differ- scientific world was startled in 1985 when Richard Smal- ent fullerene adducts with a variety of structures have ley of Rice University and Harry W.Kroto of the Univer- been prepared.Cationic derivatives of these adducts. sity of Sussex,UK,and their coworkers announced that for example,bind tightly to DNA and can be used to they had detected a new form of carbon with the mo- visualize it by electron microscopy lecular formula Coo.They suggested that the molecule An important development in this field has been the has a structure that resembles a soccer ball;it has 12 five preparation of single-wall nanotubes,which are based on membered rings and 20 six-membered rings arranged Coo or higher fullerenes and are extended for a very long such that each five-membered ring is surrounded by 5 distance to make long molecules that are hundreds of six-membered rings.This structure reminded its dis times stronger than steel and can act as molecular wires coverers of a geodesic dome,a structure invented by Nanotubes can be used as the probe (a very sharp tip) the innovative American engineer and philosopher R in atomic force microscopes,which can be used to im Buckminster Fuller.Therefore,the official name of this age single molecules.The nanotubes make the sharpest allotrope of carbon has become fullerene.Most chem- possible tips because they are of molecular dimensions. Nanotubes are also playing an increasingly important role in the field of nanotechnology,induding applica tions in the arca of molecular electronics.Nanotubes can be superconducting or semiconducting,depending on the exact structure of the nanotube. A fullerene (Gso)molecule. A nanotube
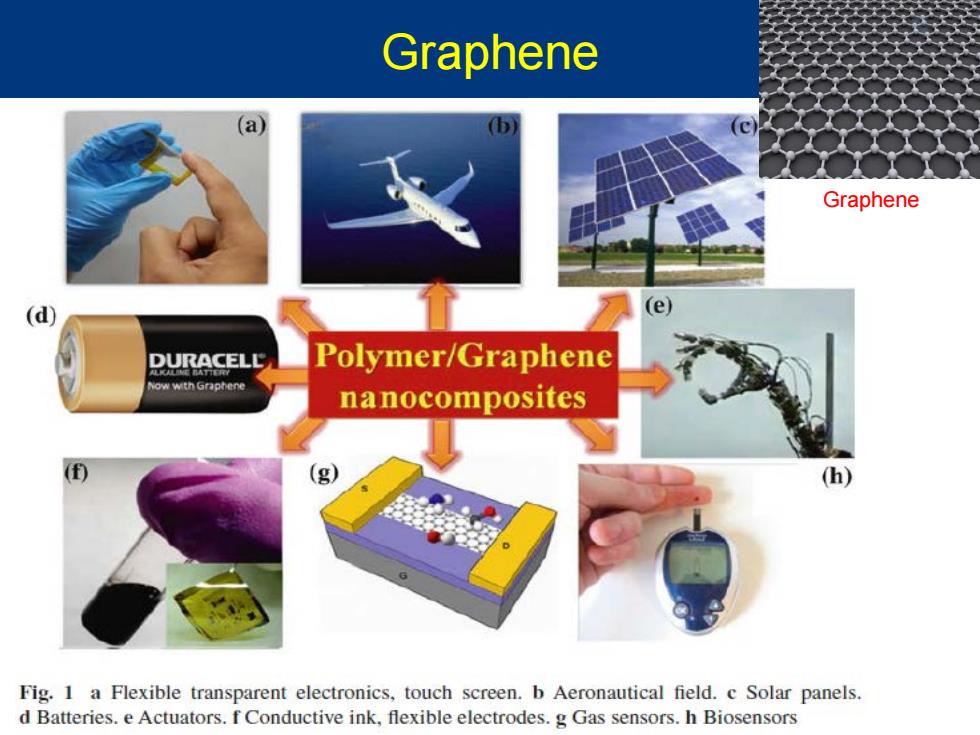
Graphene (a) (b) Graphene (d) e) DURACELL Polymer/Graphene ow wth Grapnene nanocomposites (g) (h) Fig.1 a Flexible transparent electronics,touch screen.b Aeronautical field.c Solar panels. d Batteries.e Actuators.f Conductive ink,flexible electrodes.g Gas sensors.h Biosensors
Graphene Graphene
Carbon Organic chemistry All organic compounds contain the element carbon ■4 A element Shares four electrons -Forms four strong covalent bonds Bonds to other carbons to create chains and rings Not all carbon compounds are derived from living organisms
Organic chemistry All organic compounds contain the element carbon 4A element Shares four electrons Forms four strong covalent bonds Bonds to other carbons to create chains and rings Not all carbon compounds are derived from living organisms Carbon
Really?Matter OMs The elements and their compounds represent only a small and visible fraction of the universe 4.6%!Hydrogen is the most abundant element(75%) followed by helium(23%),oxygen(1%),and then carbon(0.5%). The nature of the remainder:not well understood Dark energy (72%)and Dark matter(23%). Q?Cisotopes? Carbon has 15 (!known isotopes,from 8C to 23C,only three of which occur on Earth. 14C is the basis for"carbon dating"in archeology
The elements and their compounds represent only a small and visible fraction of the universe 4.6%!Hydrogen is the most abundant element(75%), followed by helium(23%),oxygen(1%),and then carbon (0.5%). The nature of the remainder: not well understood Dark energy (72%) and Dark matter (23%). Q? C isotopes? Carbon has 15 (!) known isotopes, from 8C to 23C, only three of which occur on Earth. 14C is the basis for “carbon dating” in archeology. Really? Matter & OMs