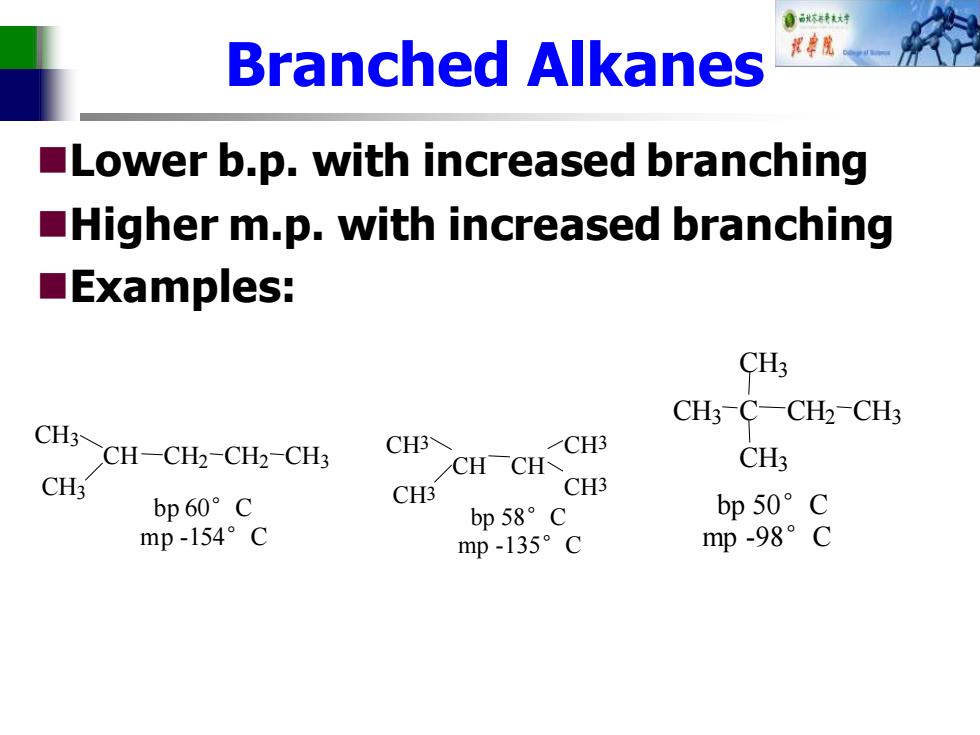
自秋东精秋对 Branched Alkanes Lower b.p.with increased branching Higher m.p.with increased branching ■Examples: CH3 CH3-C-CH2-CH3 CH3~CH-CH2-CH2-CHs CH3 CH3 CHCH CH3 CH3 CH3 CH3 bp60°C bp58°C bp50°C mp-154°C mp-135°C mp-98°C
Branched Alkanes ◼Lower b.p. with increased branching ◼Higher m.p. with increased branching ◼Examples: H CH3 CH CH3 CH2 CH2 CH3 bp 60°C mp -154°C CH3 CH CH3 CH CH3 CH3 bp 58°C mp -135°C bp 50°C mp -98°C CH3 C C 3 CH3 CH2 CH3

Major Uses of Alkanes C-C2:gases(natural gas) C3-C4:liquified petroleum(LPG) ■Cs-Cg:gasoline C-C16:diesel,kerosene,jet fuel C17-up:lubricating oils,heating oil Origin:petroleum refining
Major Uses of Alkanes ◼C1 -C2 : gases (natural gas) ◼C3 -C4 : liquified petroleum (LPG) ◼C5 -C8 : gasoline ◼C9 -C16: diesel, kerosene, jet fuel ◼C17-up: lubricating oils, heating oil ◼Origin: petroleum refining
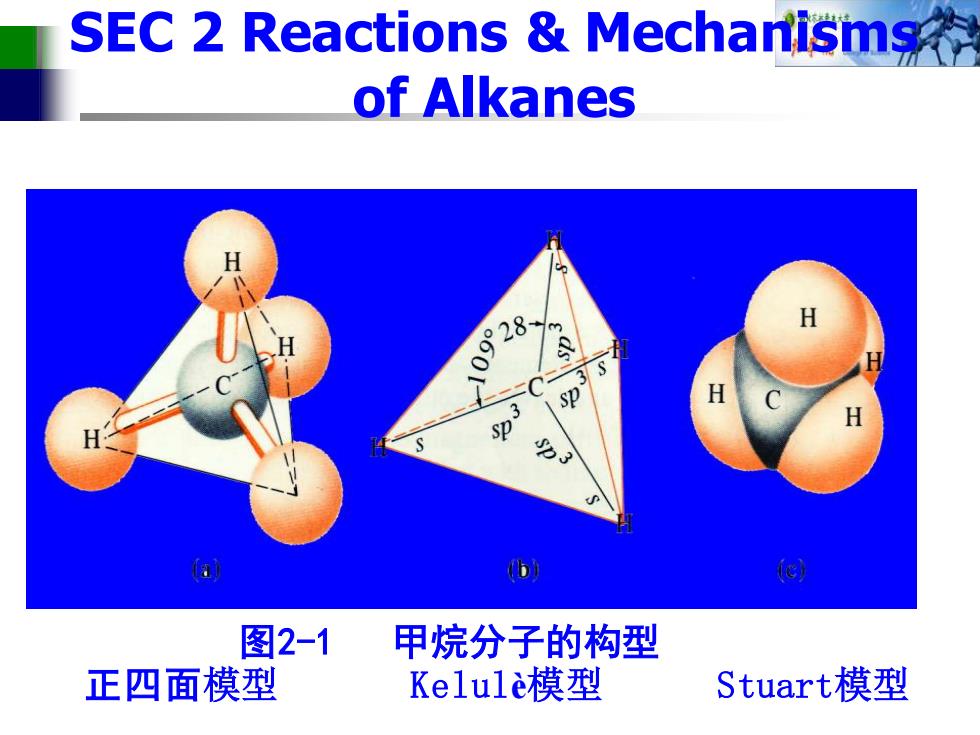
SEC 2 Reactions Mechanisms of Alkanes 0928 sp 图2-1 甲烷分子的构型 正四面模型 Kelule模型 Stuart模型
图2-1 甲烷分子的构型 正四面模型 Kelulè模型 Stuart模型 SEC 2 Reactions & Mechanisms of Alkanes
自秋转好 Structure 花单院 Alkane:single bonds,sp3 carbons Cycloalkane:carbons form a ring ■结构特征 CH3-CH3 ■SP杂化碳原子四面体碳原子 ■σ键相连 ■键合原子可绕σ键轴“自由”旋转 1874 J.H.Van't Hoff Utrecht Univ
Alkane: single bonds, sp3 carbons Cycloalkane: carbons form a ring ◼结构特征 CH3-CH3 ◼ SP3杂化碳原子 四面体碳原子 ◼ 键相连 ◼ 键合原子可绕键轴“自由”旋转 ❖1874 J.H.Van’t Hoff ❖ Utrecht Univ Structure

自秋转达对 Reactivity of alkanes Chemical stability:C-H;C-C ■不与强酸、强碱、氧化剂反应 ■自由基反应hv
Reactivity of alkanes ◼Chemical stability:C-H; C-C ◼不与强酸、强碱、氧化剂反应 ◼自由基反应 hv