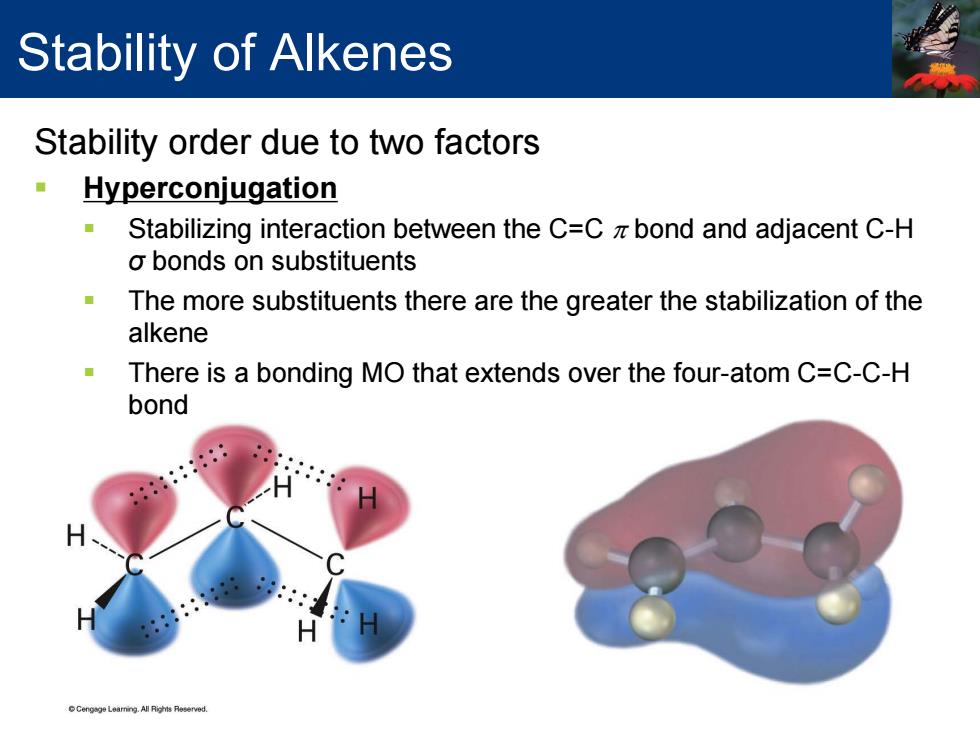
Stability of Alkenes Stability order due to two factors Hyperconjugation 年 Stabilizing interaction between the C=C z bond and adjacent C-H o bonds on substituents The more substituents there are the greater the stabilization of the alkene There is a bonding MO that extends over the four-atom C=C-C-H bond eneLeaing All Ris Reserved
Stability order due to two factors Hyperconjugation Stabilizing interaction between the C=C π bond and adjacent C-H σ bonds on substituents The more substituents there are the greater the stabilization of the alkene There is a bonding MO that extends over the four-atom C=C-C-H bond Stability of Alkenes
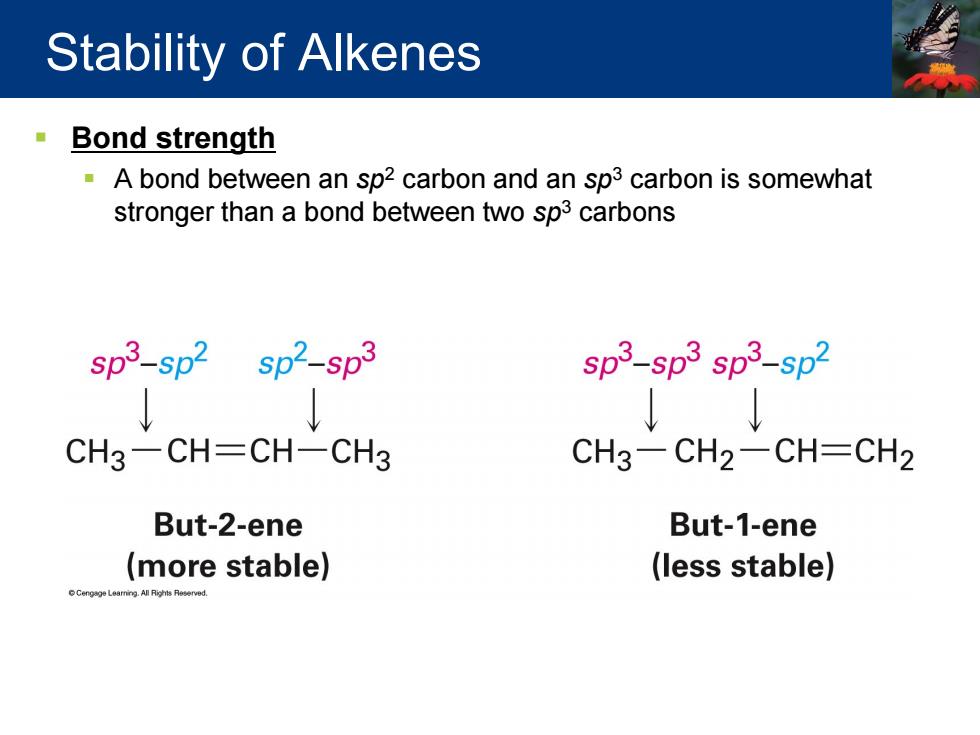
Stability of Alkenes Bond strength =A bond between an sp2 carbon and an sp3 carbon is somewhat stronger than a bond between two sp3 carbons sp3-sp2 sp2-sp3 sp3-sp3 sp3-sp2 CH3-CH-CH-CH3 CH3-CH2-CH=CH2 But-2-ene But-1-ene (more stable) (less stable)
Bond strength A bond between an sp2 carbon and an sp3 carbon is somewhat stronger than a bond between two sp3 carbons Stability of Alkenes

Sec 2 Preparation of alkenes Alkenes can be synthesized by the reduction of alkynes,or by the elimination of alkyl halides and alcohols. -E2 dehydrohalogenation (-HX); -E1 dehydrohalogenation(-HX); -Dehydration of alcohols(-H2O) -Dehalogenation of vicinal dibromides(-X2)
Sec 2 Preparation of alkenes Alkenes can be synthesized by the reduction of alkynes, or by the elimination of alkyl halides and alcohols. E2 dehydrohalogenation (-HX); E1 dehydrohalogenation (-HX); Dehydration of alcohols (-H2O) Dehalogenation of vicinal dibromides(-X2)
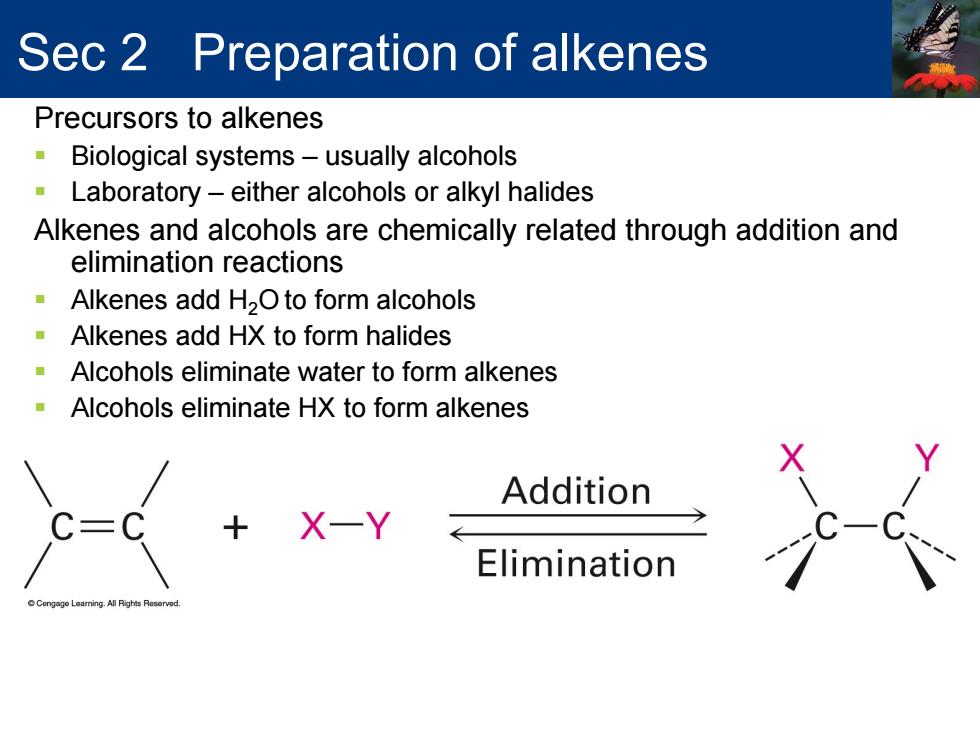
Sec 2 Preparation of alkenes Precursors to alkenes Biological systems-usually alcohols Laboratory-either alcohols or alkyl halides Alkenes and alcohols are chemically related through addition and elimination reactions Alkenes add H2O to form alcohols Alkenes add HX to form halides -Alcohols eliminate water to form alkenes Alcohols eliminate HX to form alkenes Addition C=( X-Y Elimination a.All Riahts Ros
Precursors to alkenes Biological systems – usually alcohols Laboratory – either alcohols or alkyl halides Alkenes and alcohols are chemically related through addition and elimination reactions Alkenes add H2O to form alcohols Alkenes add HX to form halides Alcohols eliminate water to form alkenes Alcohols eliminate HX to form alkenes Sec 2 Preparation of alkenes
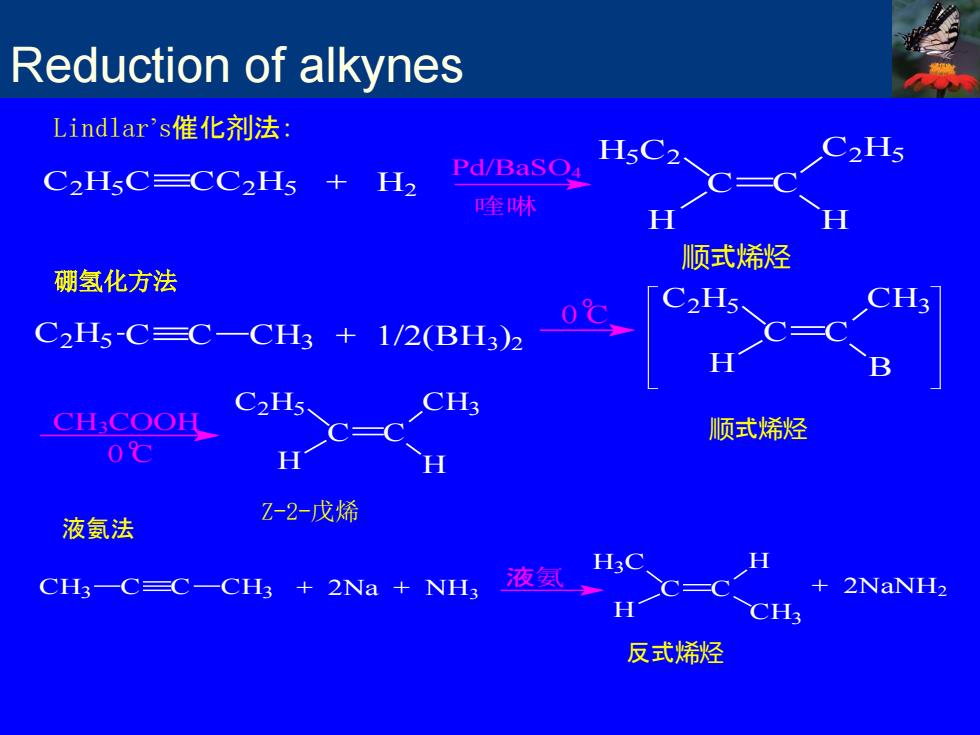
Reduction of alkynes Lindlar's催化剂法: C2H5 C2H5C=CC2H5 H2 Pd/BaSO 喹林 顺式烯烃 硼氢化方法 C2H5-C=C-CH3+1/2(BH3)2 C2H5 CH;COOH 顺式烯烃 0℃ H 液氨法 Z-2-戊烯 CH3—C=C-CH3+2Na+NH3 液氨 + 2NaNH2 反式烯烃
顺式烯烃 C2H5C CC2H5 + H2 Pd/BaSO4 喹啉 C C H5C2 C2H5 H H Lindlar’s催化剂法: 硼氢化方法 C2H5 C C CH3 + 1/2(BH3)2 o 0 C C C C2H5 CH3 H B o 0 C CH3COOH C C C2H5 CH3 H H Z-2-戊烯 顺式烯烃 Reduction of alkynes CH3 C C CH3 + 2Na + NH3 液氨 C C + 2NaNH2 H H CH3 H3C 反式烯烃 液氨法