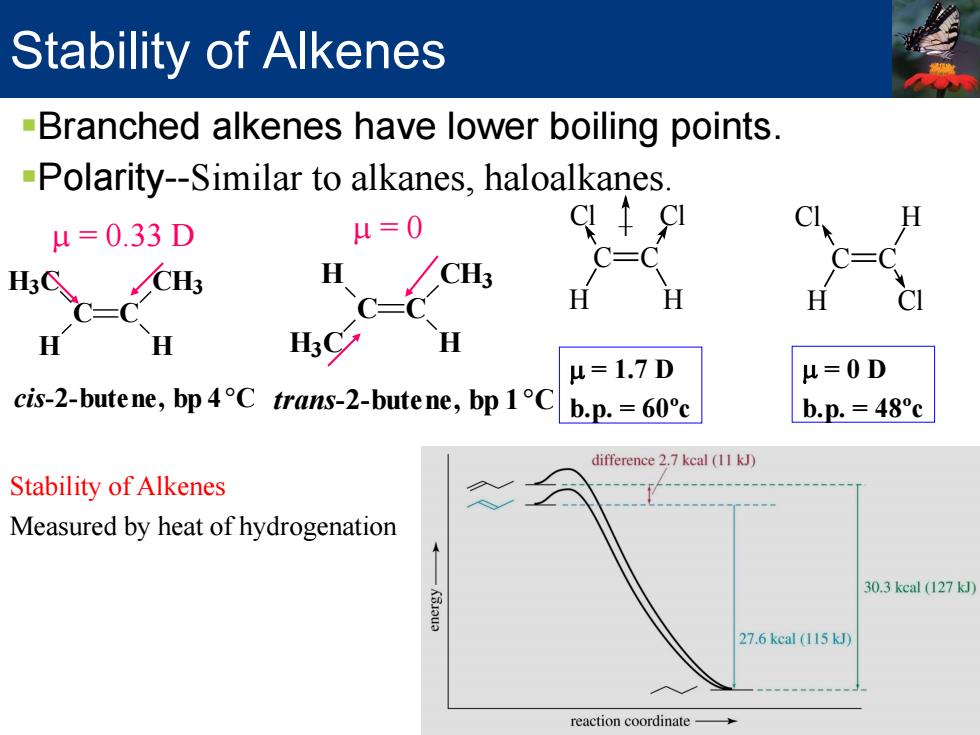
Stability of Alkenes -Branched alkenes have lower boiling points. -Polarity--Similar to alkanes,haloalkanes. μ=0.33D u=0 c-c CH3 H CH3 H H u=1.7D H=0D cis-2-butene,bp4C trans-2-butene,bp 1C b.p.=60c b.p.=48c difference 2.7 kcal (11 kJ) Stability of Alkenes Measured by heat of hydrogenation 30.3kcal(127k 27.6 kcal (115 kJ) reaction coordinate-
Stability of Alkenes Branched alkenes have lower boiling points. Polarity--Similar to alkanes, haloalkanes. Cl C H C H Cl Cl C H C Cl H µ = 1.7 D b.p. = 60ºc µ = 0 D b.p. = 48ºc µ = 0.33 D cis-2-butene, bp 4°C C C H H3C H CH3 µ = 0 trans-2-butene, bp 1°C C C H H H3C CH3 Stability of Alkenes Measured by heat of hydrogenation
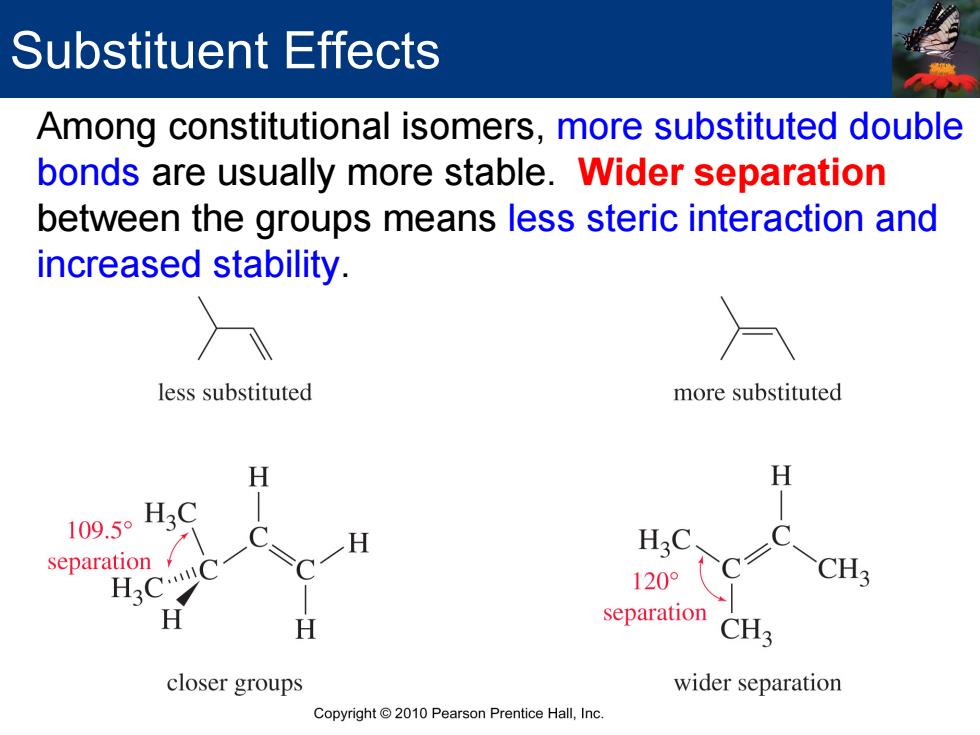
Substituent Effects Among constitutional isomers,more substituted double bonds are usually more stable.Wider separation between the groups means less steric interaction and increased stability. less substituted more substituted H 109.5° H3C separation H3 .tl 120° H separation CH3 closer groups wider separation Copyright 2010 Pearson Prentice Hall,Inc
Substituent Effects Among constitutional isomers, more substituted double bonds are usually more stable. Wider separation between the groups means less steric interaction and increased stability
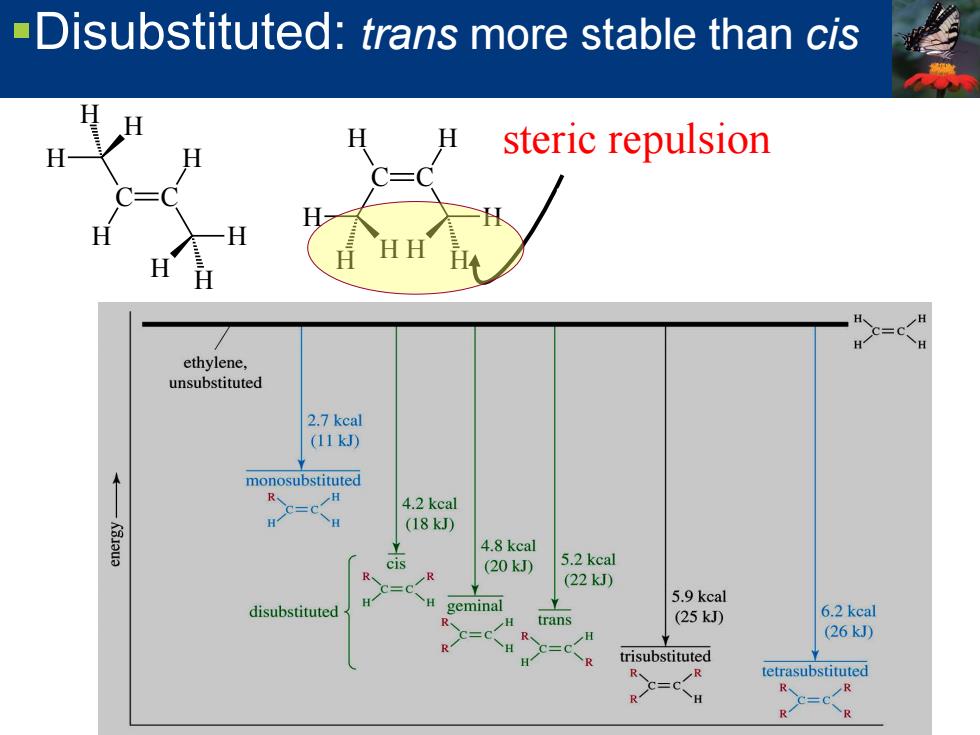
-Disubstituted:trans more stable than cis steric repulsion ethylene, unsubstituted 2.7 kcal (11kJ) monosubstituted R 4.2 kcal H (18kJ) K8Jeua 4.8 kcal cis (20kJ) 5.2 kcal R (22kJ) =0 H 5.9 kcal disubstituted geminal trans (25kD 6.2 kcal c=c R (26kJ) C=c R trisubstituted c=c R、 tetrasubstituted R H R、 CR R R
Disubstituted: trans more stable than cis steric repulsion C C H H H H H H H H C C H H H H H H H H Relative Stabilities
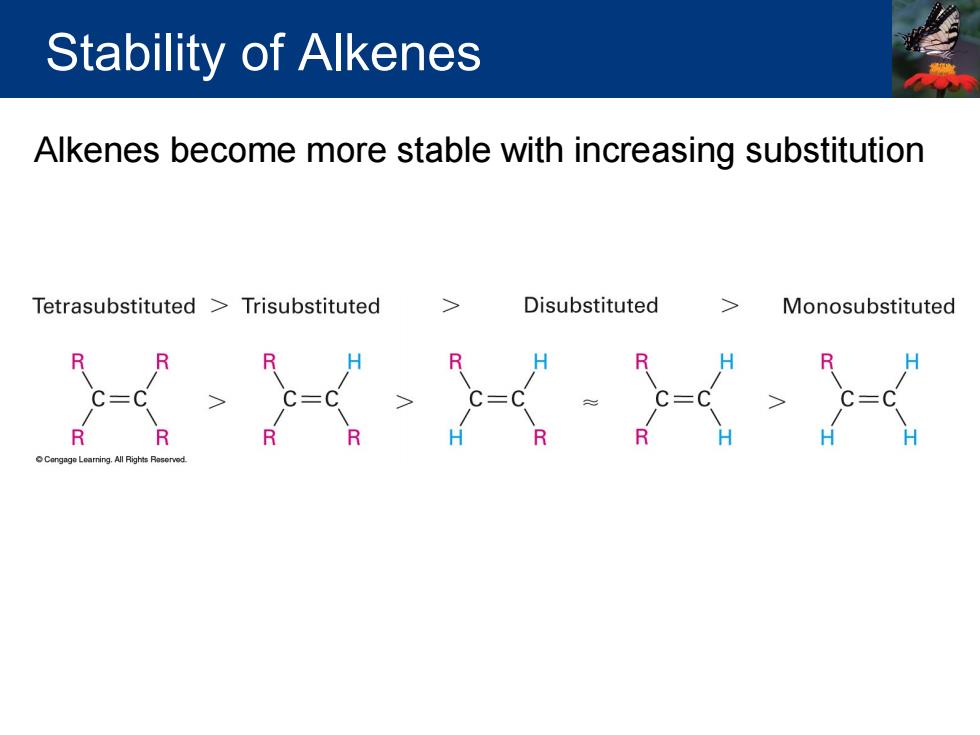
Stability of Alkenes Alkenes become more stable with increasing substitution Tetrasubstituted Trisubstituted Disubstituted Monosubstituted R R R
Alkenes become more stable with increasing substitution Stability of Alkenes
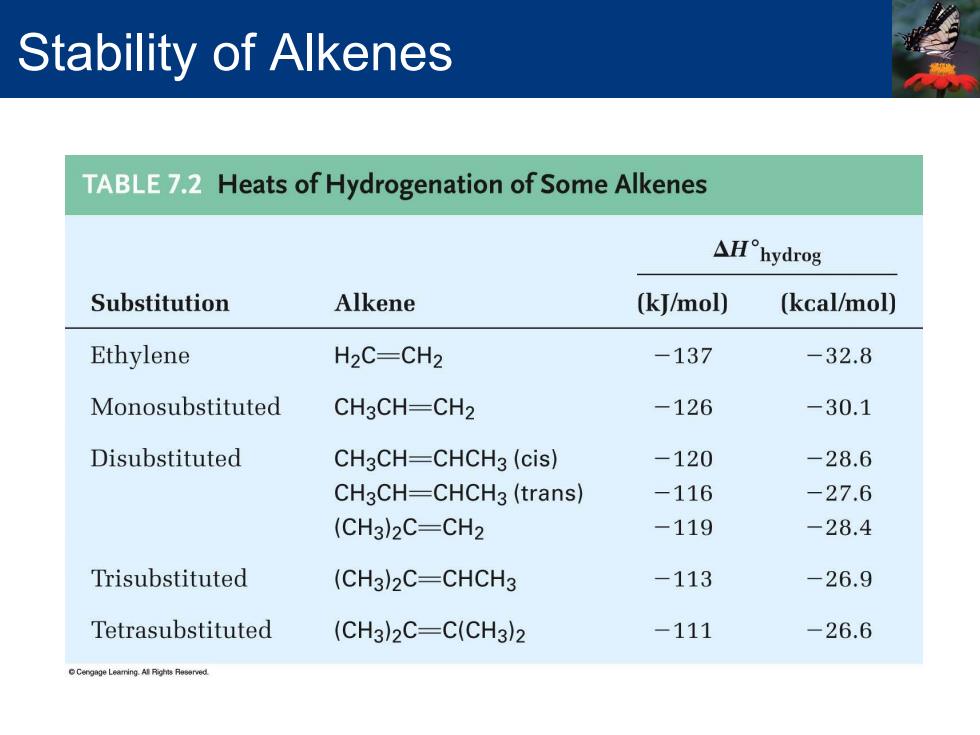
Stability of Alkenes TABLE72 Heats of Hydrogenation of Some Alkenes △H°hydrog Substitution Alkene (kJ/mol) (kcal/mol) Ethylene H2C=CH2 -137 -32.8 Monosubstituted CH3CH=CH2 -126 -30.1 Disubstituted CH3CH=CHCH3(cis) -120 -28.6 CH3CH=CHCH3(trans) -116 -27.6 (CH3)2C=CH2 -119 -28.4 Trisubstituted (CH3)2C=CHCH3 -113 -26.9 Tetrasubstituted (CH3)2C-C(CH3)2 -111 -26.6 Cengge LearringARhts Reserved
Stability of Alkenes