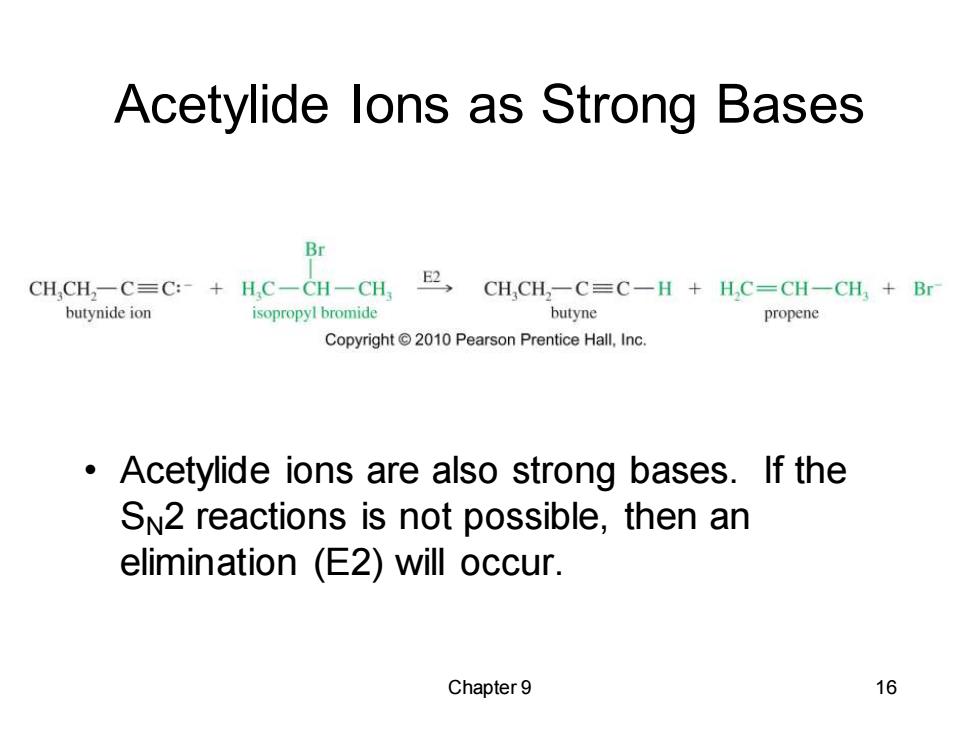
Acetylide lons as Strong Bases Br CH,CH,-C=C:-+HC-CH-CH,E,CH,CH:-C=C-H H.C-CH-CH,Br butynide ion isopropyl bromide butyne propene Copyright2010 Pearson Prentice Hall,Inc. Acetylide ions are also strong bases.If the SN2 reactions is not possible,then an elimination (E2)will occur. Chapter 9 16
Chapter 9 16 Acetylide Ions as Strong Bases • Acetylide ions are also strong bases. If the SN2 reactions is not possible, then an elimination (E2) will occur

Solved Problem 1 Show how to synthesize 3-decyne from acetylene and any necessary alkyl halides. Solution Another name for 3-decyne is ethyl n-hexylacetylene.It can be made by adding an ethyl group and a hexyl group to acetylene.This can be done in either order;we begin by adding the hexyl group. H一C=C一H (1)NaNH2 (2)CH:(CHa)sBr CH3(CH2)5一C=C一H acetylene I-octyne CH3(CH2)5-C=C-H (1NaNH,:→ (2)CH:CH2Br CH3(CH2)s-C=C-CH2CH3 I-octyne 3-decyne Chapter 9 17
Chapter 9 17 Show how to synthesize 3-decyne from acetylene and any necessary alkyl halides. Another name for 3-decyne is ethyl n-hexylacetylene. It can be made by adding an ethyl group and a hexyl group to acetylene. This can be done in either order; we begin by adding the hexyl group. Solved Problem 1 Solution