
Organic Chemistry,7th Edition L.G.Wade,Jr. Chapter 6 Alkyl Halides:Nucleophilic Substitution and Elimination Copyright 2010 Pearson Education,Inc
Chapter 6 Organic Chemistry, 7th Edition L. G. Wade, Jr. Alkyl Halides: Nucleophilic Substitution and Elimination Copyright © 2010 Pearson Education, Inc
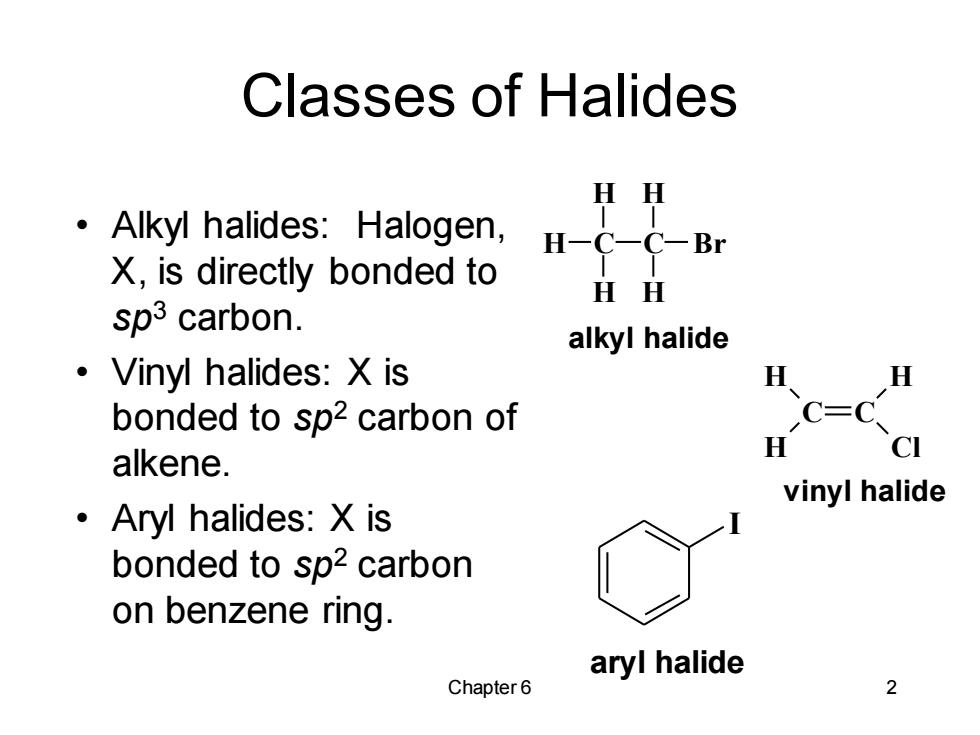
Classes of Halides HH Alkyl halides:Halogen, H-C一C-Br X,is directly bonded to HH sp3 carbon. alkyl halide ·Vinyl halides:Xis H H bonded to sp2 carbon of C=C H CI alkene. vinyl halide ·Aryl halides:Xis bonded to sp2 carbon on benzene ring. aryl halide Chapter 6 2
Chapter 6 2 Classes of Halides • Alkyl halides: Halogen, X, is directly bonded to sp3 carbon. • Vinyl halides: X is bonded to sp2 carbon of alkene. • Aryl halides: X is bonded to sp2 carbon on benzene ring. C C H H H Cl vinyl halide C H H H C H H Br alkyl halide I aryl halide
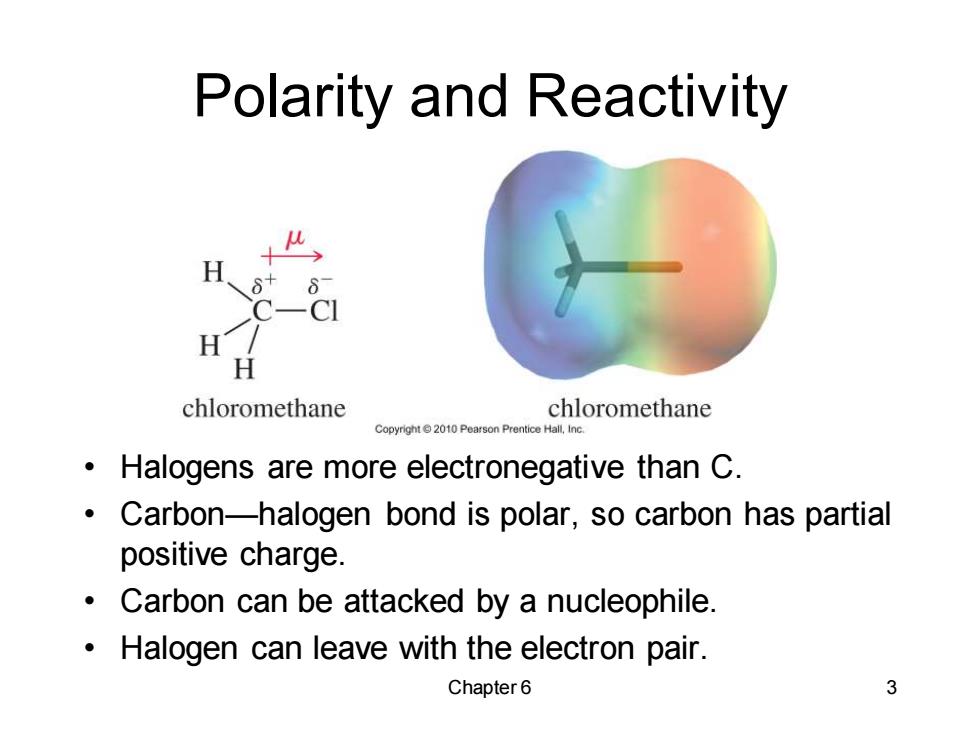
Polarity and Reactivity H 6+ -CI chloromethane chloromethane Halogens are more electronegative than C. Carbon-halogen bond is polar,so carbon has partial positive charge. Carbon can be attacked by a nucleophile. Halogen can leave with the electron pair. Chapter6 3
Chapter 6 3 Polarity and Reactivity • Halogens are more electronegative than C. • Carbon—halogen bond is polar, so carbon has partial positive charge. • Carbon can be attacked by a nucleophile. • Halogen can leave with the electron pair
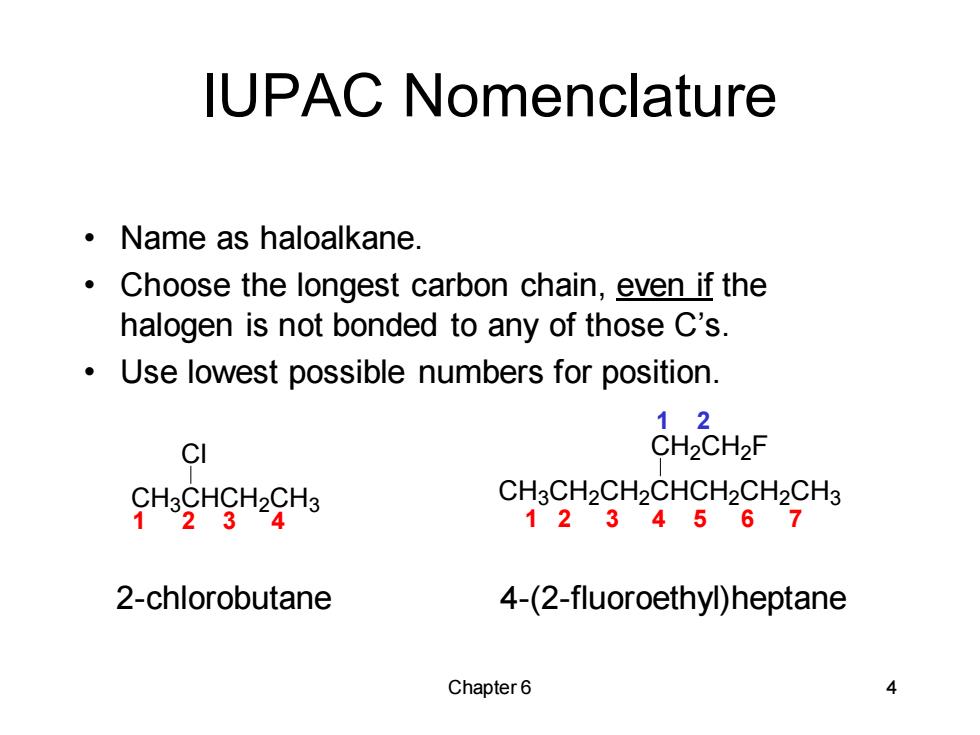
IUPAC Nomenclature 。Name as haloalkane. Choose the longest carbon chain,even if the halogen is not bonded to any of those C's. Use lowest possible numbers for position. 12 CI CH2CH2F HgHgHH CH3CH2CH2CHCH2CH2CH3 1234567 2-chlorobutane 4-(2-fluoroethyl)heptane Chapter6 4
Chapter 6 4 IUPAC Nomenclature • Name as haloalkane. • Choose the longest carbon chain, even if the halogen is not bonded to any of those C’s. • Use lowest possible numbers for position. CH3CH2CH2CHCH2CH2CH3 CH2CH2 F 1 2 3 4 2-chlorobutane 4-(2-fluoroethyl)heptane 1 2 3 4 5 6 7 1 2 CH3CHCH2CH3 Cl
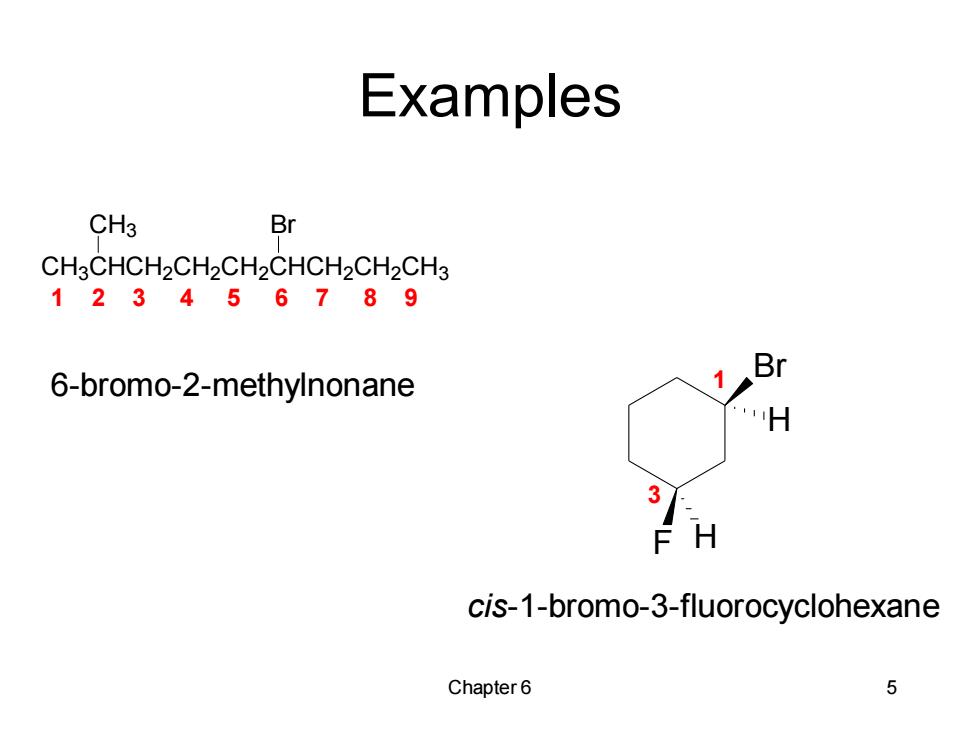
Examples CH3 Br CH3CHCH2CH2CH2CHCH2CH2CH3 123456789 Br 6-bromo-2-methylnonane H 3 F H cis-1-bromo-3-fluorocyclohexane Chapter6 5
Chapter 6 5 Examples CH3CHCH2CH2CH2CHCH2CH2CH3 CH3 Br Br F H H 1 2 3 4 5 6 7 8 9 6-bromo-2-methylnonane 1 3 cis-1-bromo-3-fluorocyclohexane