
a)The pH Electrode This is a device for measuring the concentration of hydrogen ions and hence the degree of acidity of a solution since pH is defined as the negative logarithm of the hydrogen ion concentration;
a) The pH Electrode ◼ This is a device for measuring the concentration of hydrogen ions and hence the degree of acidity of a solution - since pH is defined as the negative logarithm of the hydrogen ion concentration;
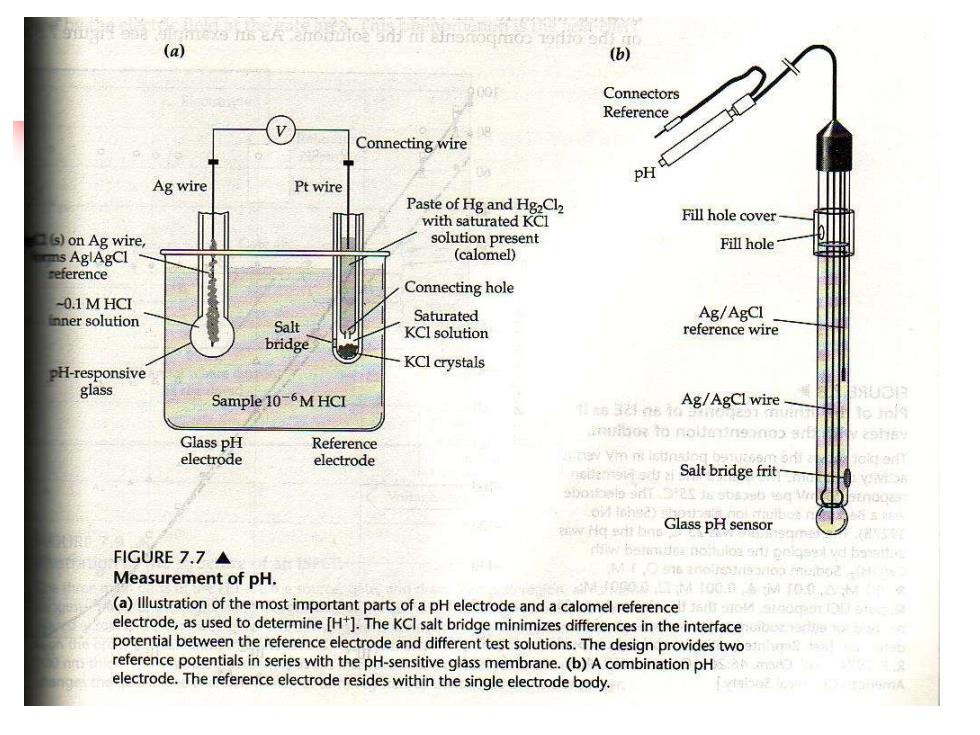
(a) (b) Connectors Reference Connecting wire Ag wire Pt wire Paste of Hg and Hg2Cl2 with saturated KCl Fill hole cover s)on Ag wire solution present Fill hole- ns AglAgCI (calomel) eference Connecting hole -0.1MHCI nner solution Saturated Ag/AgCI Salt KCl solution reference wire bridge H-responsive KClcrystals glass Sample 10-6M HCI Ag/AgCl wire- Glass pH Reference electrode electrode Salt bridge frit Glass pH sensor FIGURE7.7▲ Measurement of pH 0在 (a)llustration of the most important parts of a pH electrode and a calomel reference electrode,as used to determine [H+].The KCl salt bridge minimizes differences in the interface potential between the reference electrode and different test solutions.The design provides two reference potentials in series with the pH-sensitive glass membrane.(b)A combination pH electrode.The reference electrode resides within the single electrode body
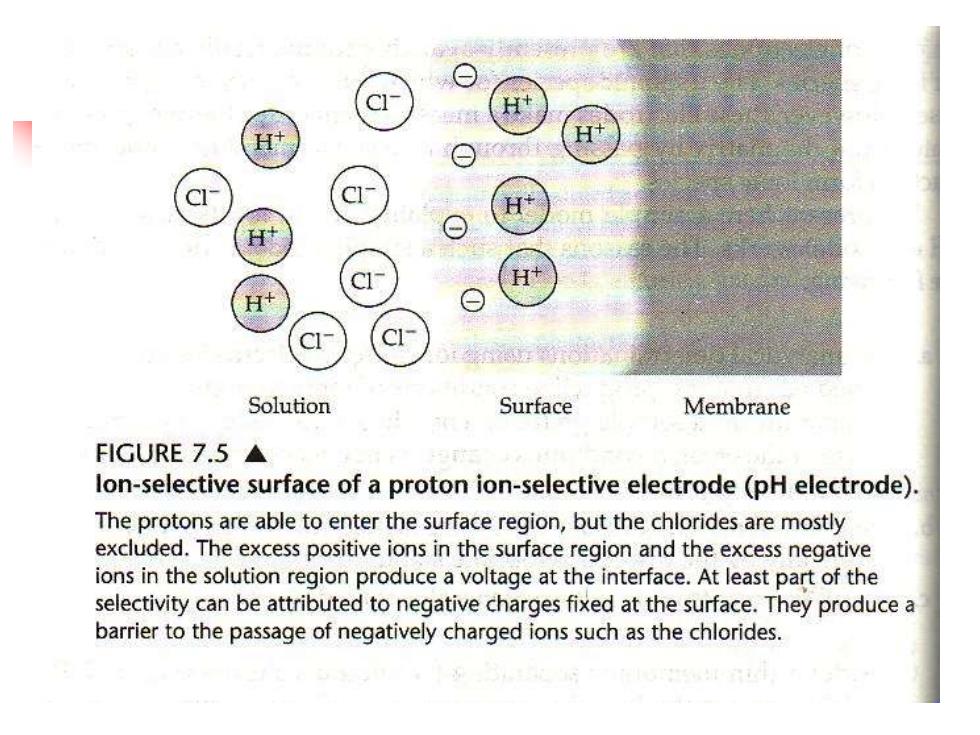
Solution Surface Membrane FIGURE7.5▲ lon-selective surface of a proton ion-selective electrode(pH electrode). The protons are able to enter the surface region,but the chlorides are mostly excluded.The excess positive ions in the surface region and the excess negative ions in the solution region produce a voltage at the interface.At least part of the selectivity can be attributed to negative charges fixed at the surface.They produce a barrier to the passage of negatively charged ions such as the chlorides

The most essential component of a pH electrode is a special,sensitive glass membrane which permits the passage of hydrogen ions,but no other ionic species When the electrode is immersed in a test solution containing hydrogen ions the external ions diffuse through the membrane until an equilibrium is reached between the external and internal concentrations.Thus there is a build up of charge on the inside of the membrane which is proportional to the number of hydrogen ions in the external solution
◼ The most essential component of a pH electrode is a special, sensitive glass membrane which permits the passage of hydrogen ions, but no other ionic species. When the electrode is immersed in a test solution containing hydrogen ions the external ions diffuse through the membrane until an equilibrium is reached between the external and internal concentrations. Thus there is a build up of charge on the inside of the membrane which is proportional to the number of hydrogen ions in the external solution

The potential difference developed across the membrane is in fact directly proportional to the Logarithm of the ionic concentration in the external solution. Thus,in order to determine the pH of an unknown solution,it is only necessary to measure the potential difference in two standard solutions of known pH,construct a straight line calibration graph by plotting millivolts versus pH (=Log [H+])then read off the unknown pH from the measured voltage
◼ The potential difference developed across the membrane is in fact directly proportional to the Logarithm of the ionic concentration in the external solution. Thus, in order to determine the pH of an unknown solution, it is only necessary to measure the potential difference in two standard solutions of known pH, construct a straight line calibration graph by plotting millivolts versus pH (= - Log [H+ ]) then read off the unknown pH from the measured voltage