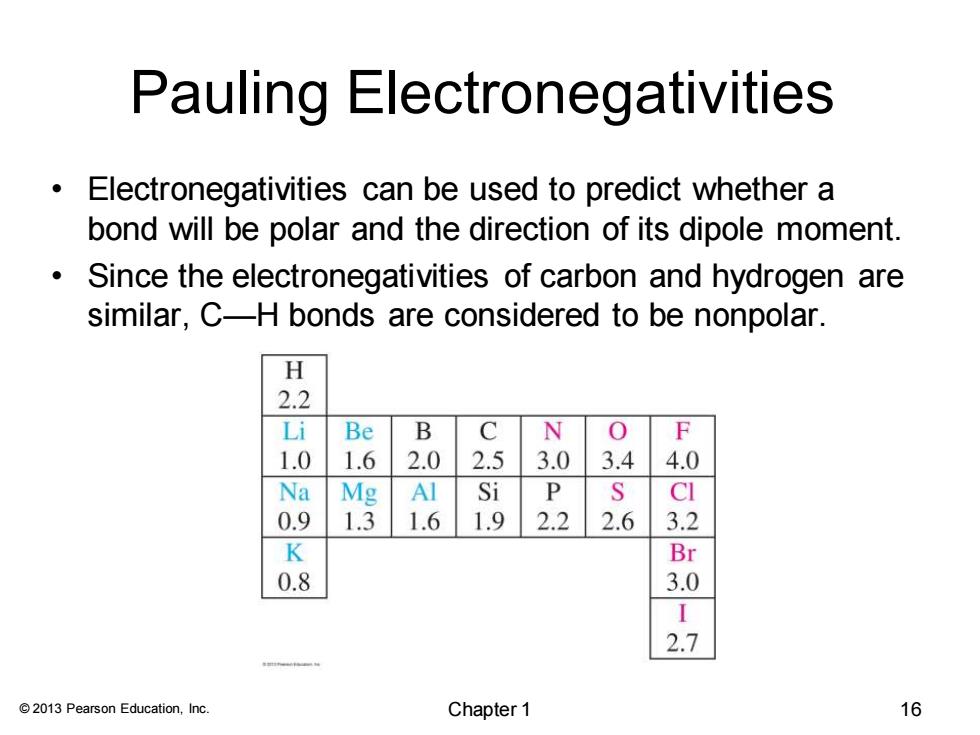
Pauling Electronegativities Electronegativities can be used to predict whether a bond will be polar and the direction of its dipole moment. Since the electronegativities of carbon and hydrogen are similar,C-H bonds are considered to be nonpolar. H 2.2 Li Be B C N 0 F 1.0 1.6 2.0 2.5 3.0 3.4 4.0 Na Mg Al Si P S CI 0.9 1.3 1.6 1.9 2.2 2.6 3.2 K Br 0.8 3.0 2.7 2013 Pearson Education,Inc. Chapter 1 16
© 2013 Pearson Education, Inc. Pauling Electronegativities • Electronegativities can be used to predict whether a bond will be polar and the direction of its dipole moment. • Since the electronegativities of carbon and hydrogen are similar, C—H bonds are considered to be nonpolar. Chapter 1 16

Formal Charges Formal charge [group number ]-[nonbonding electrons ]-2[shared electrons] H30+ NO+ 6-2-2(6)=+1 6-2-2(6)=+1 H-ǒ-H ,N三O: 5-2-h(6)=0 Formal charges are a way of keeping track of electrons. They may or may not correspond to actual charges in the molecule. 2013 Pearson Education,Inc. Chapter 1 17
© 2013 Pearson Education, Inc. Formal Charges H3O+ NO+ H O H H Formal charge = [group number ] – [nonbonding electrons ] – ½ [shared electrons] N O 6 – 2 – ½ (6) = +1 6 – 2 – ½ (6) = +1 5 – 2 – ½ (6) = 0 + + • Formal charges are a way of keeping track of electrons. • They may or may not correspond to actual charges in the molecule. Chapter 1 17