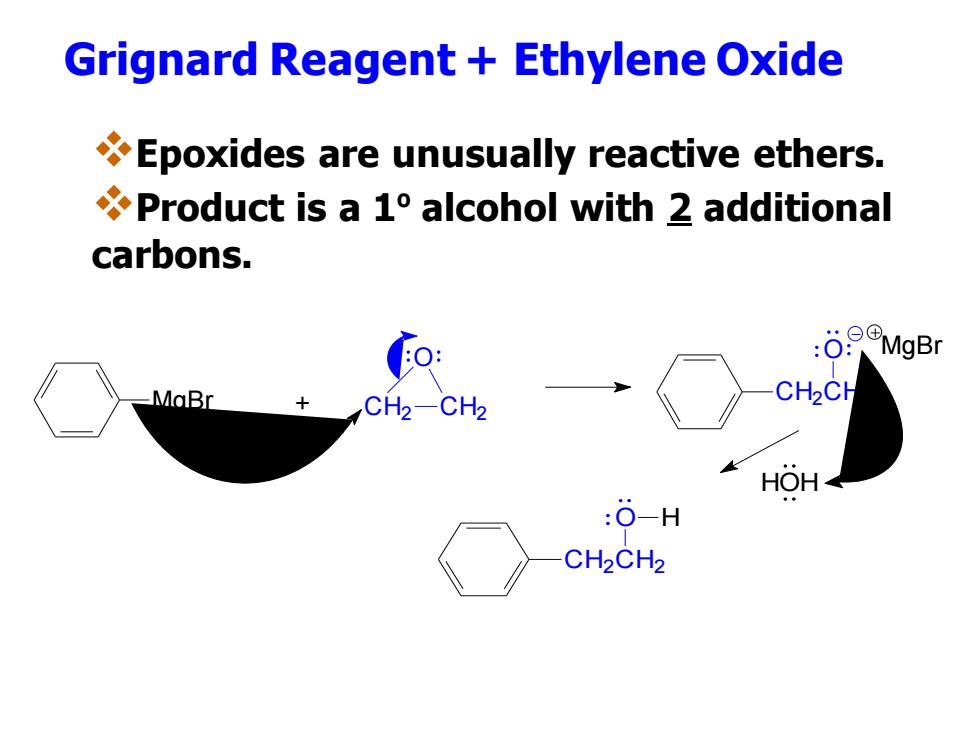
Grignard Reagent Ethylene Oxide Epoxides are unusually reactive ethers. Product is a 1 alcohol with 2 additional carbons. -MaBr CH2 CH2CH HOH :O-H CH2CH2
Grignard Reagent + Ethylene Oxide ❖Epoxides are unusually reactive ethers. ❖Product is a 1ºalcohol with 2 additional carbons. MgBr + CH2 CH2 O CH2CH2 O MgBr HOH CH2CH2 O H

2.Reduction of Carbonyl Reduction of aldehyde yields 1 alcohol. Reduction of ketone yields 2 alcohol. Reagents: -Sodium borohydride,NaBH4 Lithium aluminum hydride,LiAlH4 -Raney nickel
2. Reduction of Carbonyl ❖Reduction of aldehyde yields 1ºalcohol. ❖Reduction of ketone yields 2ºalcohol. ❖Reagents: ◼Sodium borohydride, NaBH4 ◼Lithium aluminum hydride, LiAlH4 ◼Raney nickel

CHO CH2OH + 4CHOH NaBH CH3OII 4 NaOCH3 +B(OCH3) 96% CH3 CH3 OH LiOH +Al(OH)3 90% 0 c-0+N0aa时,一→mgoN+aa Mee rwein-Poundorf反应 R
CH3 CHO 4 + 4CH3OH + NaBH4 CH3OH 4 CH2OH CH3 + NaOCH3 + B(OCH3) 3 96% O 4 + LiAlH4 乙醚 H2O 4 H OH + LiOH + Al(OH) 3 90% C R R O + Al OCH(CH3) 2 3 (RCHO) 3Al + CH3CCH3 R O H3O RCHOH Meerwein- Poundorf 反应 R
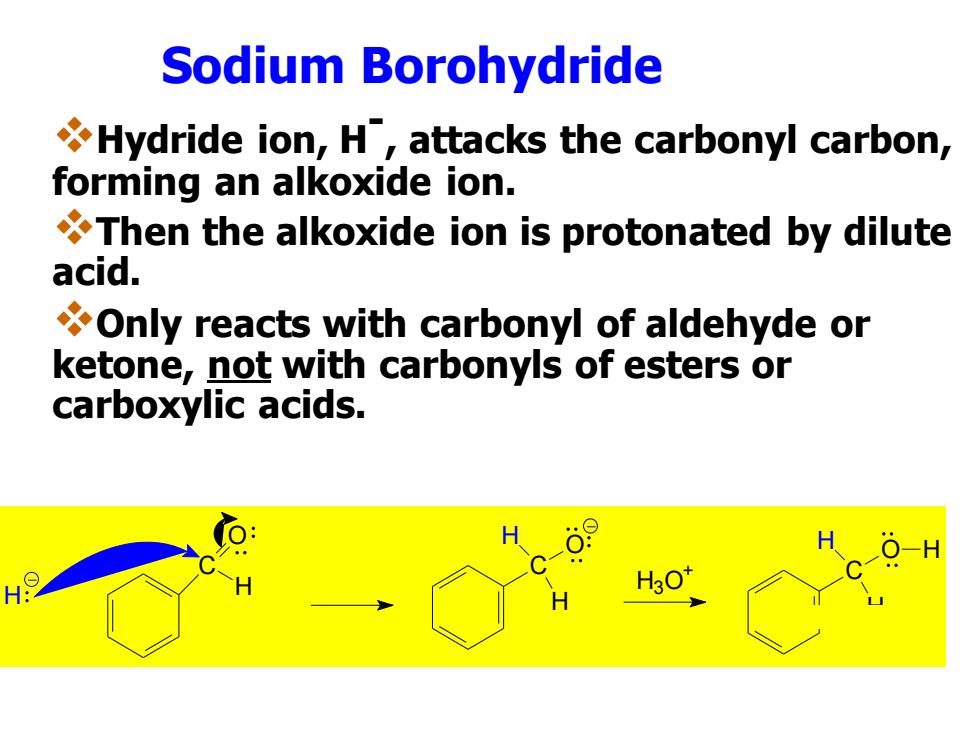
Sodium Borohydride Hydride ion,H,attacks the carbonyl carbon, forming an alkoxide ion. Then the alkoxide ion is protonated by dilute acid. Only reacts with carbonyl of aldehyde or ketone,not with carbonyls of esters or carboxylic acids. 30
Sodium Borohydride ❖Hydride ion, H- , attacks the carbonyl carbon, forming an alkoxide ion. ❖Then the alkoxide ion is protonated by dilute acid. ❖Only reacts with carbonyl of aldehyde or ketone, not with carbonyls of esters or carboxylic acids. H C O H C H H O C H H O H H3O +

Lithium Aluminum Hydride Stronger reducing agent than sodium borohydride,but dangerous to work with. Converts esters and acids to 1 alcohols. 二0 -H C-OCHs LAH H3O* H
Lithium Aluminum Hydride ❖Stronger reducing agent than sodium borohydride, but dangerous to work with. ❖Converts esters and acids to 1º alcohols. C O OCH3 C H O H H H3O + LAH