
Carbonyl Structure HC、 0=: c-0: H HC Formaldehyde Acetone 4=2.27D 4=2.88D Electrostatic Potential Map Negatively polarized Positively oxygen polarized carbon 丙酮
Carbonyl Structure 丙酮 Electrostatic Potential Map Positively polarized carbon Negatively polarized oxygen
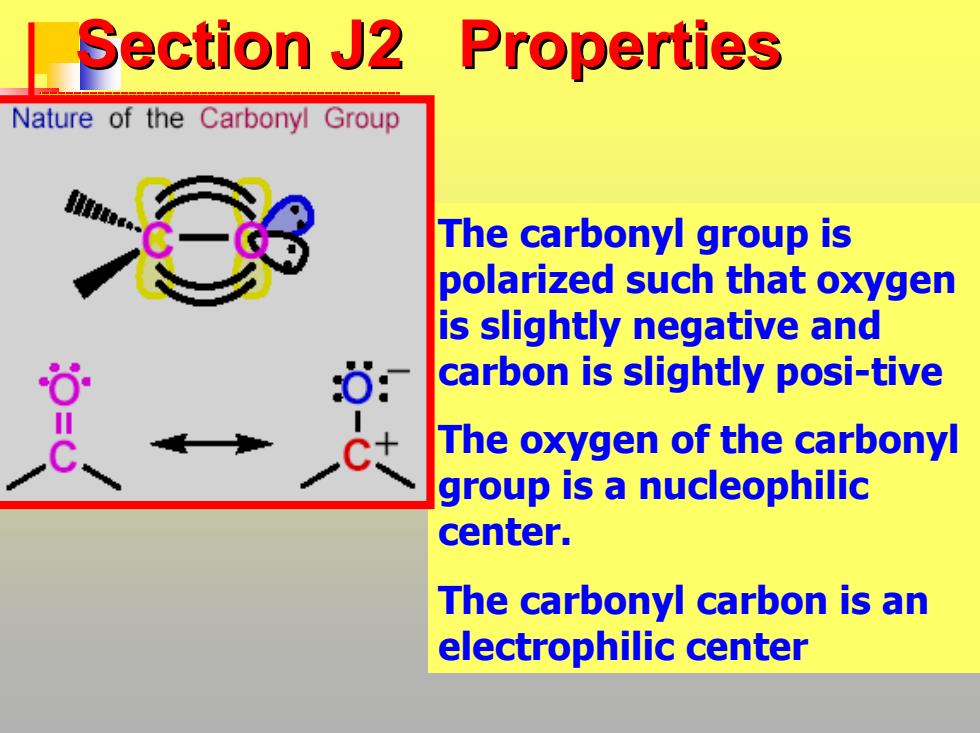
Section J2 Properties Nature of the Carbonyl Group The carbonyl group is polarized such that oxygen is slightly negative and carbon is slightly posi-tive The oxygen of the carbonyl group is a nucleophilic center. The carbonyl carbon is an electrophilic center
Section J2 Properties Section J2 Properties The carbonyl group is polarized such that oxygen is slightly negative and carbon is slightly posi-tive The oxygen of the carbonyl group is a nucleophilic center. The carbonyl carbon is an electrophilic center
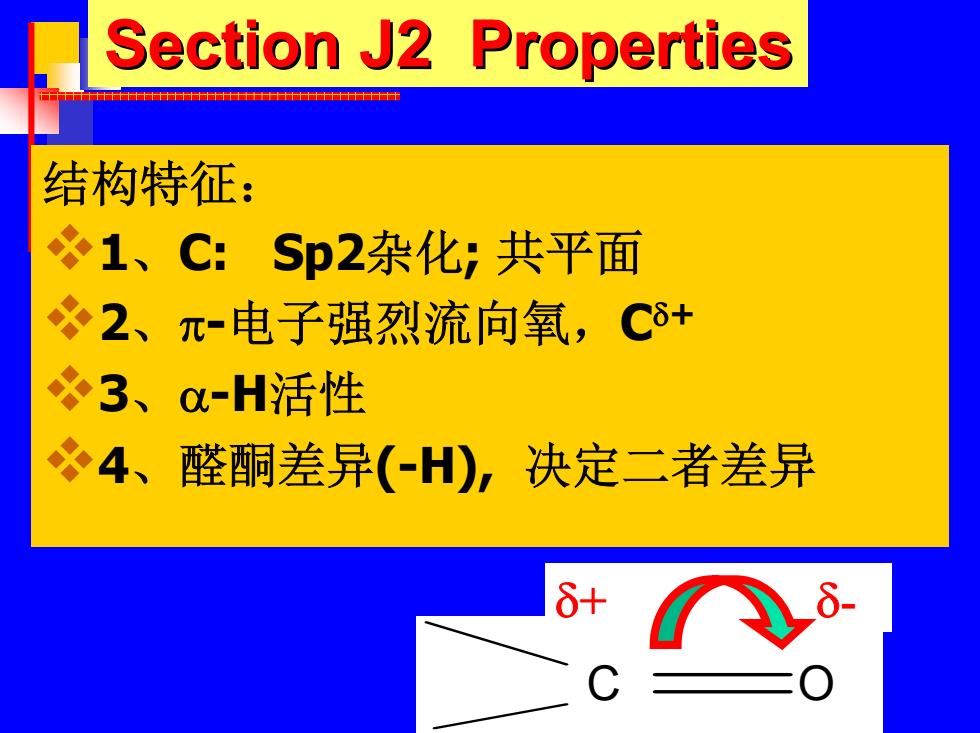
Section J2 Properties 结构特征: 1、C:Sp2杂化,共平面 必2、π-电子强烈流向氧,C8+ 3、0-H活性 必4、醛酮差异(-H),决定二者差异
Section J2 Properties Section J2 Properties 结构特征: 1 、C: Sp2杂化; 共平面 2 、 π -电子强烈流向氧, C δ + 3 、 α-H活性 4、醛酮差异(-H), 决定二者差异 C O δ+ δ -
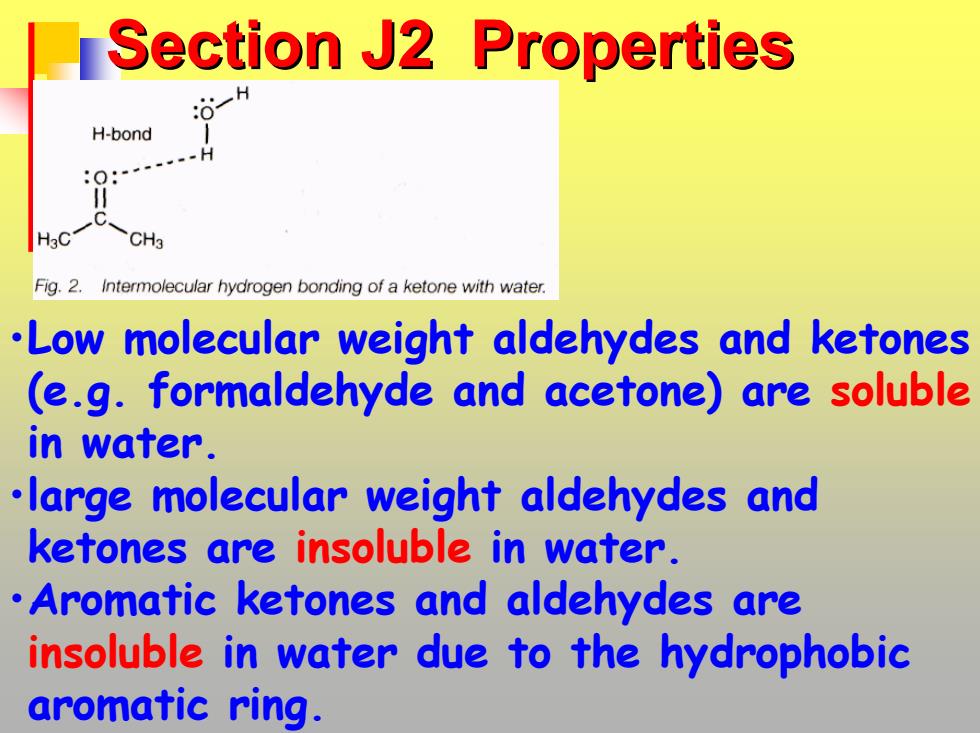
Section J2 Properties H-bond CH3 Fig.2.Intermolecular hydrogen bonding of a ketone with water. .Low molecular weight aldehydes and ketones (e.g.formaldehyde and acetone)are soluble in water. large molecular weight aldehydes and ketones are insoluble in water. Aromatic ketones and aldehydes are insoluble in water due to the hydrophobic aromatic ring
Section J2 Properties Section J2 Properties •Low molecular weight aldehydes and ketones (e.g. formaldehyde and acetone) are soluble in water. •large molecular weight aldehydes and ketones are insoluble in water. •Aromatic ketones and aldehydes are insoluble in water due to the hydrophobic aromatic ring
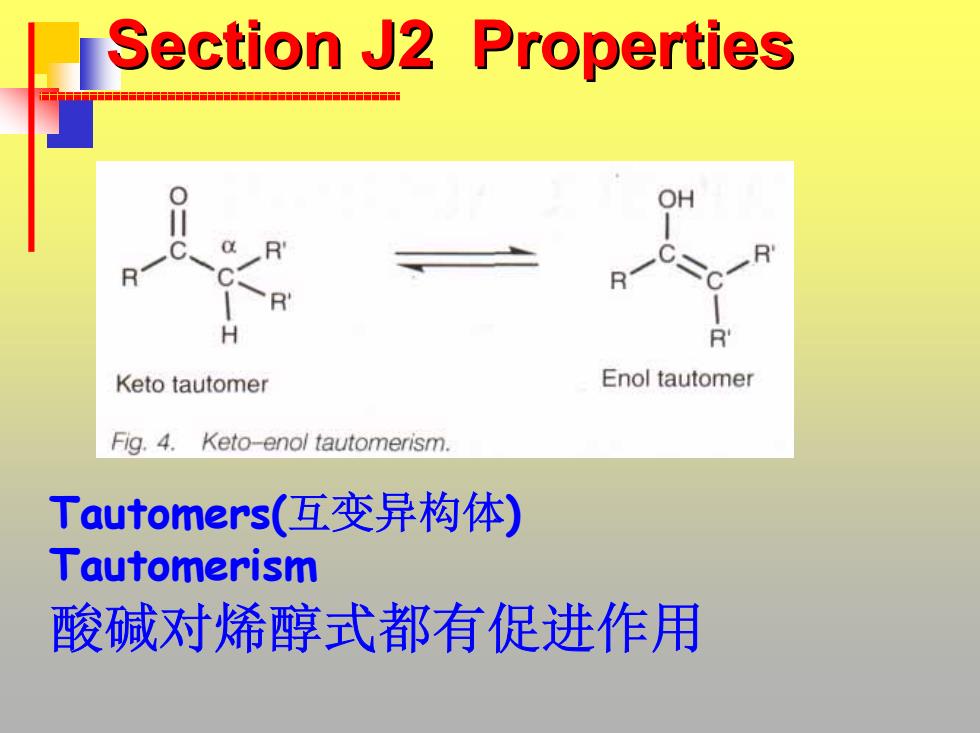
Section J2 Properties R R H Keto tautomer Enol tautomer Fig.4.Keto-enol tautomerism. Tautomers(互变异构体) Tautomerism 酸碱对烯醇式都有促进作用
Section J2 Properties Section J2 Properties Tautomers(互变异构体 ) Tautomerism 酸碱对烯醇式都有促进作用