
Section J2 Properties 十h十am ⊕ H Step 1 Step2 :O-H H Fig.5.Acid-catalyzed mechanism for keto-enol tautomerism
Section J2 Properties Section J2 Properties
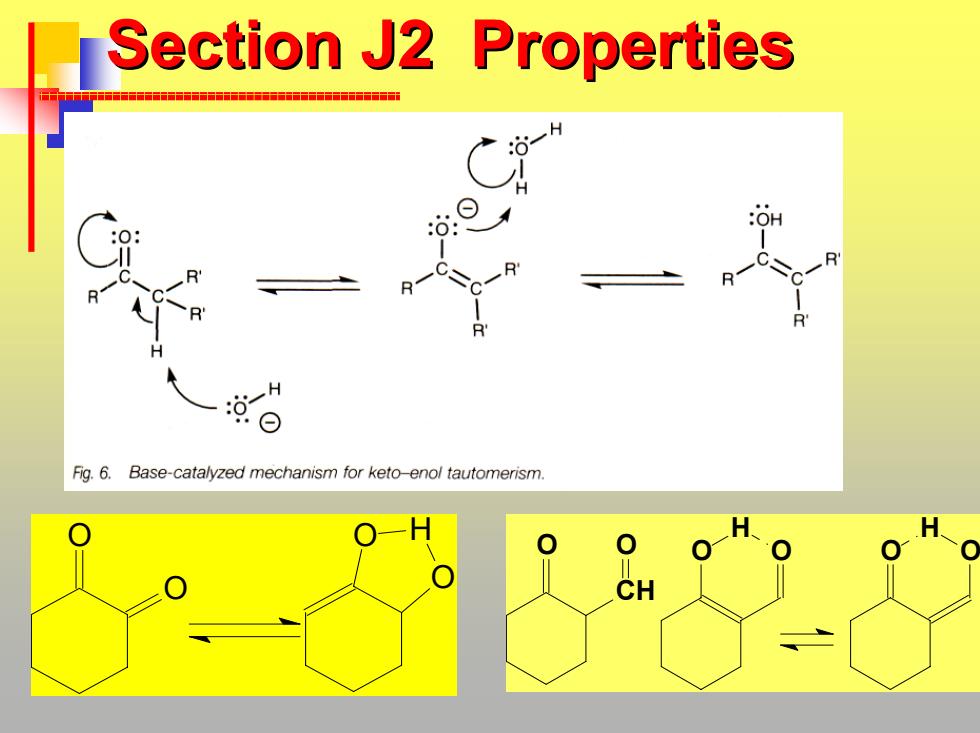
Section J2 Properties H Fig.6.Base-catalyzed mechanism for keto-enol tautomerism. CH
Section J2 Properties Section J2 Properties O O O H O CH O O O H O O H O
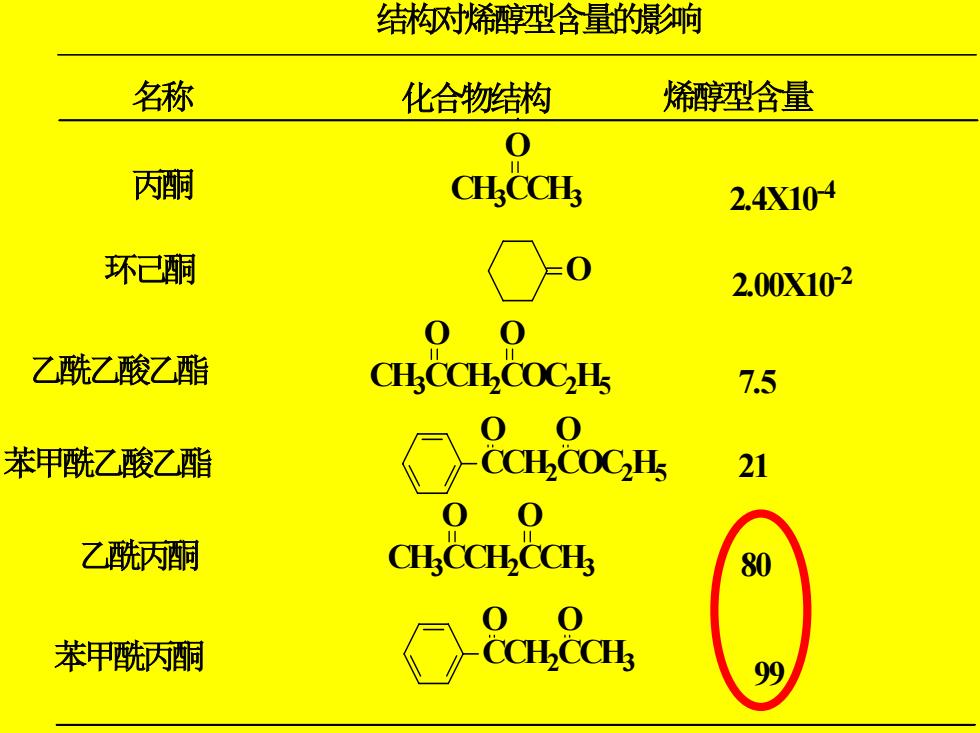
结构对烯醇型含量的影响 名称 化合物结构 烯醇型含量 0 丙酮 CH.CCH 2.4X104 环己酮 0 2.00X102 0 0 乙酰乙酸乙酯 CH.CCH COCH 7.5 00 苯甲酰乙酸乙酯 CCHCOCH5 21 00 乙酰丙酮 CH马CCHCCH 80 00 苯甲酰丙酮 CCH.CCH
结构对烯醇型含量的影响 名称 化合物结构 烯醇型含量 丙酮 环己酮 乙酰乙酸乙酯 苯甲酰乙酸乙酯 乙酰丙酮 苯甲酰丙酮 CH3CCH3 O O CH3CCH2COC2 H5 O O CCH2COC2 H5 O O CH3CCH2CCH3 O O CCH2CCH3 O O 2.4X10-4 2.00X10-2 7. 5 21 80 99

Keto Tautomer Enol Tautomer Cnol Tautomer 0 pKa=9 pure liquid water solution hexane solution H2 CH3 76 20 92 2,4pentanedione 0 0 =11 CH3 -% ethyacet如aett▣ % 8 04 46 0K,=1 01 0 1 diethyl malonate
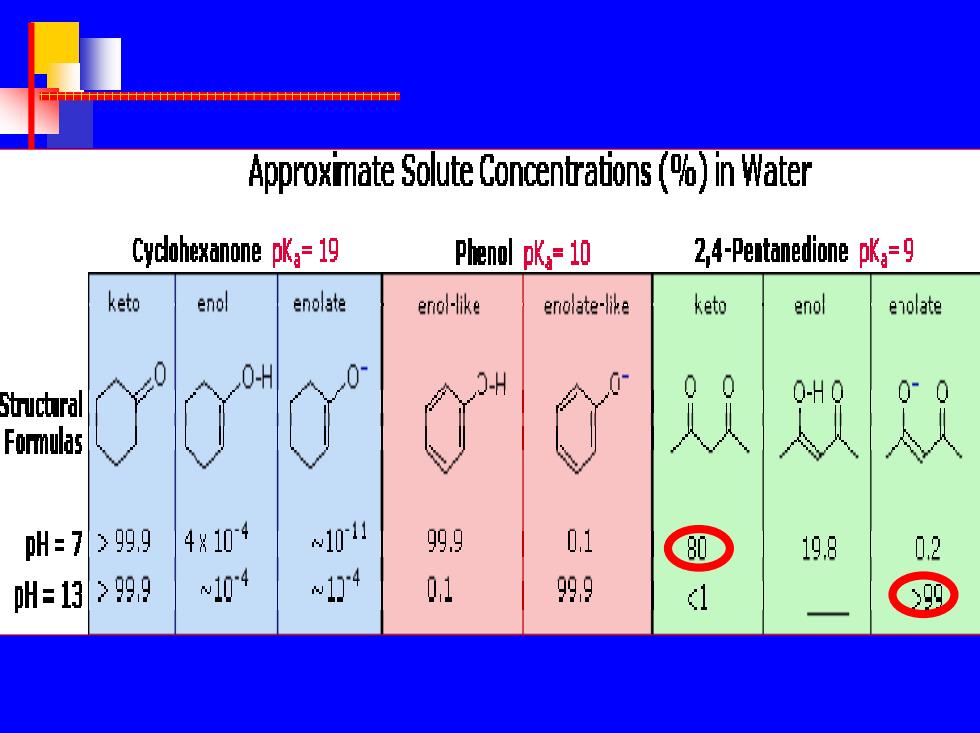
h于十十十十 Approximate Solute Concentrations(%)in Water Cyclohexanone pK=19 Phenol pK,=10 2,4-Peatanedione pKa=9 keto enol enolate eno-ike enolate-like keto enol enolate 0- Structral 0-H0 0°0 Formulas l=7399.9 4g104 ~1011 99,9 0.1 19.8 0.2 M=13>999 w104 1 01 99,9 d 驷