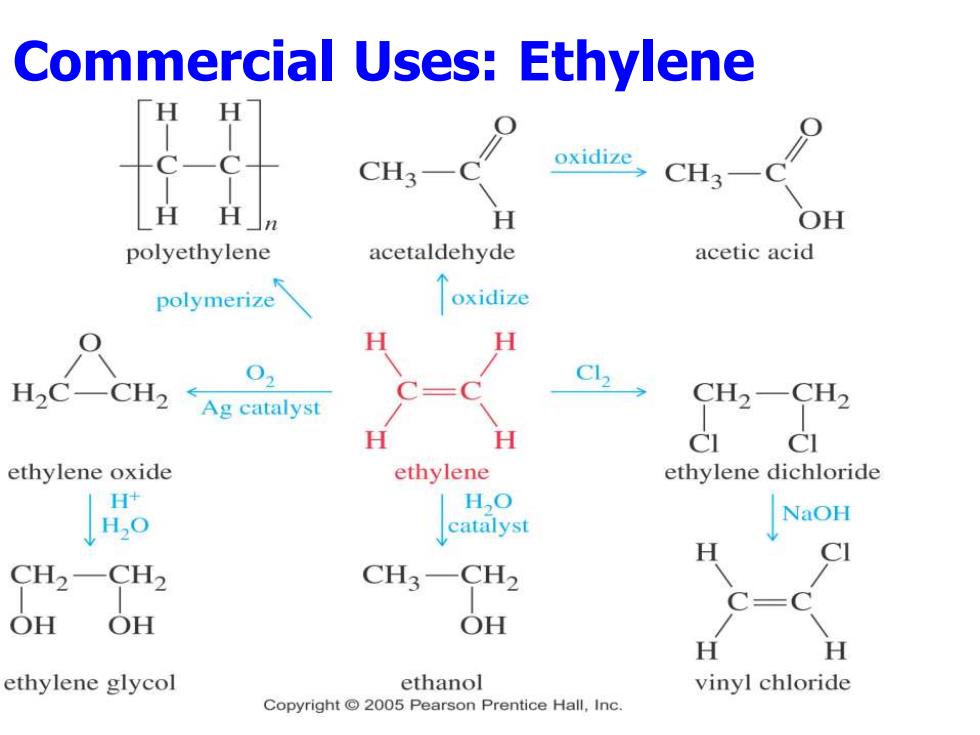
Commercial Uses:Ethylene H H CH3- oxidize CH3- H H」n OH polyethylene acetaldehyde acetic acid polymerize oxidize H H H2CCH2←Ag catalyst C12 CH2-CH2 H H CI ethylene oxide ethylene ethylene dichloride H+ H,O HO NaOH catalyst CI CH2-CH2 CH3-CH2 OH OH OH H ethylene glycol ethanol vinyl chloride Copyright 2005 Pearson Prentice Hall,Inc
Commercial Uses: Ethylene
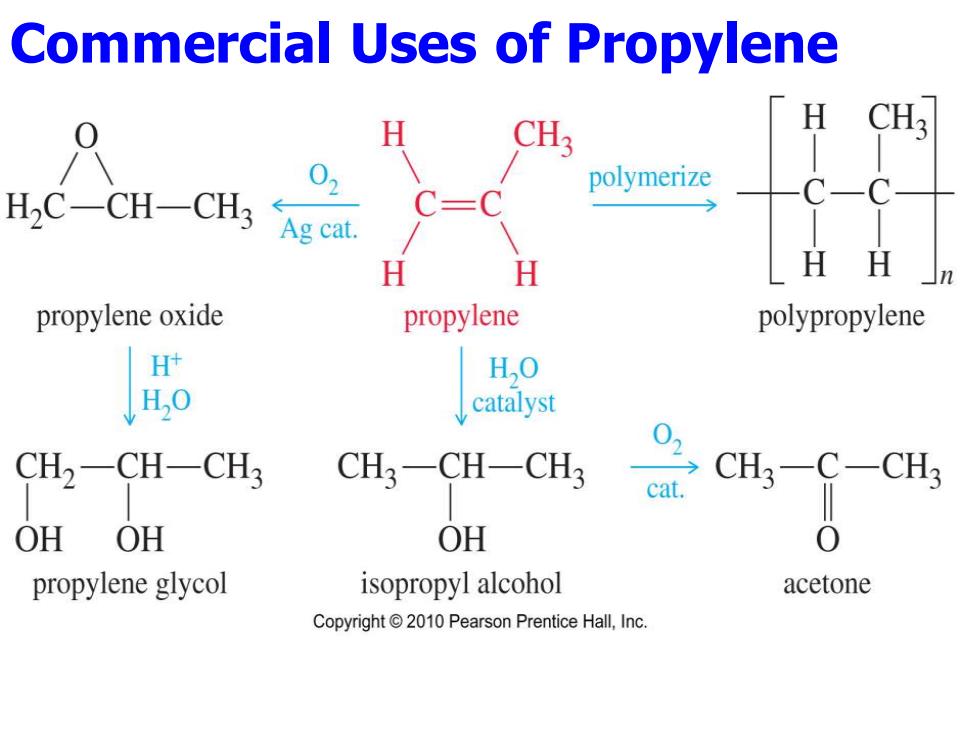
Commercial Uses of Propylene H 02 HC-CH-CH3 Ag cat polymerize H H HH propylene oxide propylene polypropylene H H,O H,0 catalyst CH2-CH-CH3 CH3一CH-CH cat. CH3-C-CH3 OH OH OH 0 propylene glycol isopropyl alcohol acetone Copyright 2010 Pearson Prentice Hall,Inc
Commercial Uses of Propylene
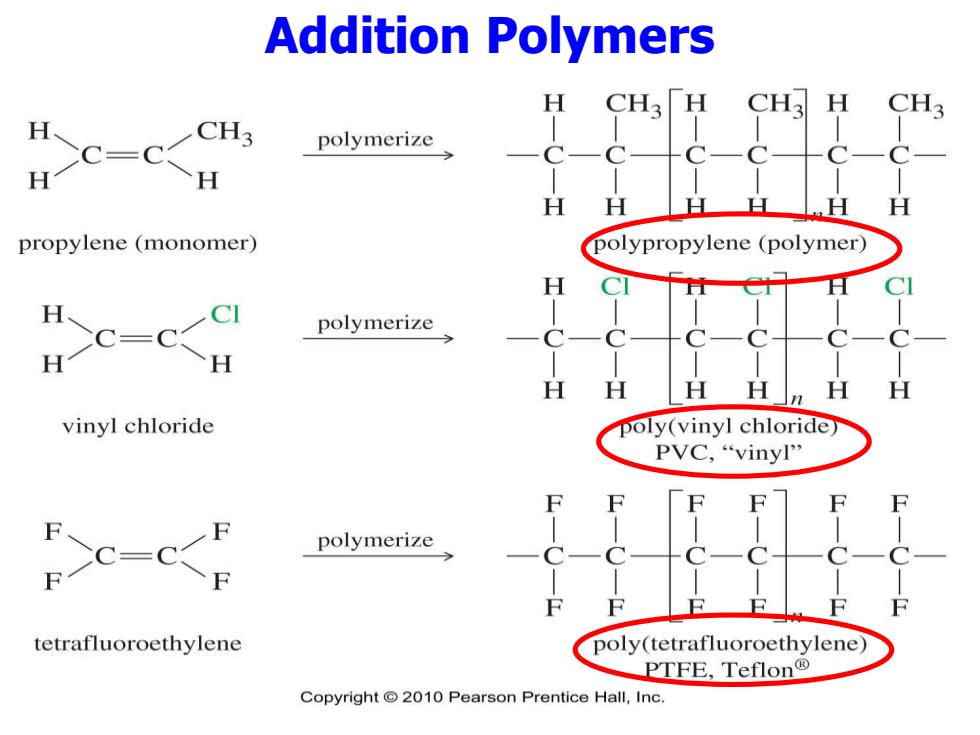
Addition Polymers H CH3「H CH3 H CH3 CH3 H CC-CCH polymerize H H propylene(monomer) polypropylene (polymer) H C=C polymerize H H H n vinyl chloride poly(vinyl chloride PVC,“vinyl'' polymerize tetrafluoroethylene poly(tetrafluoroethylene) PTFE,Teflon® Copyright 2010 Pearson Prentice Hall,Inc
Addition Polymers
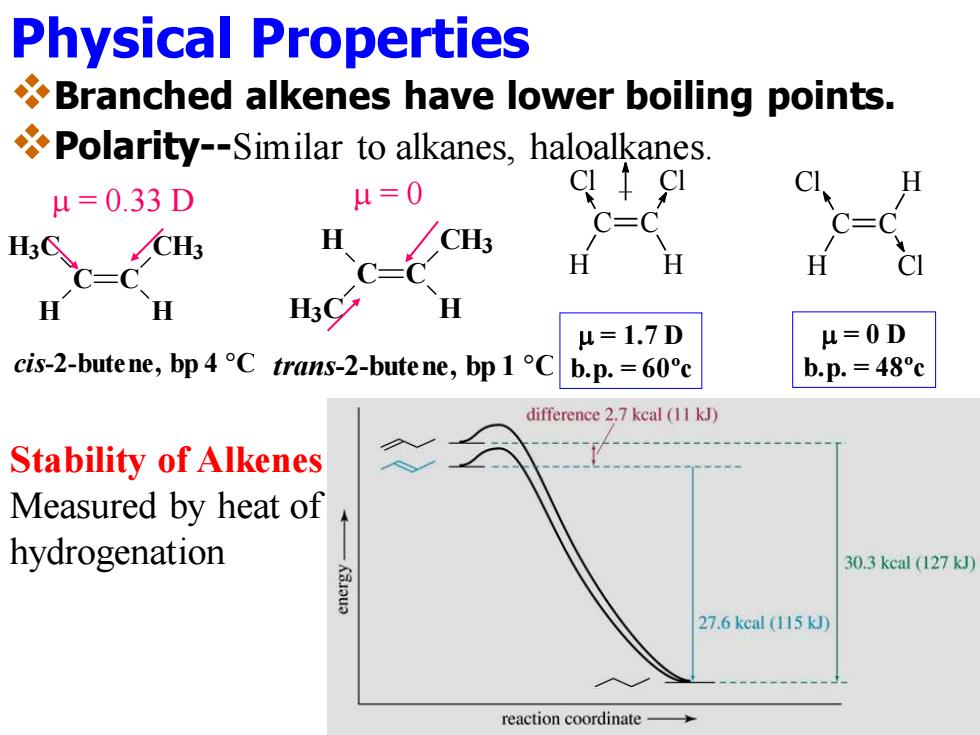
Physical Properties Branched alkenes have lower boiling points. Polarity--Similar to alkanes,haloalkanes. u=0 H u=0.33D C=C H CH3 C=C H H 人交 H H u=1.7D H=0D cis-2-butene,bp 4 C trans-2-butene,bp 1 Cb.p.=60c b.p.=48c difference 2.7 kcal (11 kJ) Stability of Alkenes Measured by heat of hydrogenation 30.3 kcal (127 kJ) 27.6kcal(115kJ) reaction coordinate
Physical Properties ❖Branched alkenes have lower boiling points. ❖Polarity--Similar to alkanes, haloalkanes. Cl C H C H Cl Cl C H C Cl H m = 1.7 D b.p. = 60ºc m = 0 D b.p. = 48ºc m = 0.33 D cis-2-butene, bp 4 °C C C H H3C H CH3 m = 0 trans-2-butene, bp 1 °C C C H H H3C CH3 Stability of Alkenes Measured by heat of hydrogenation
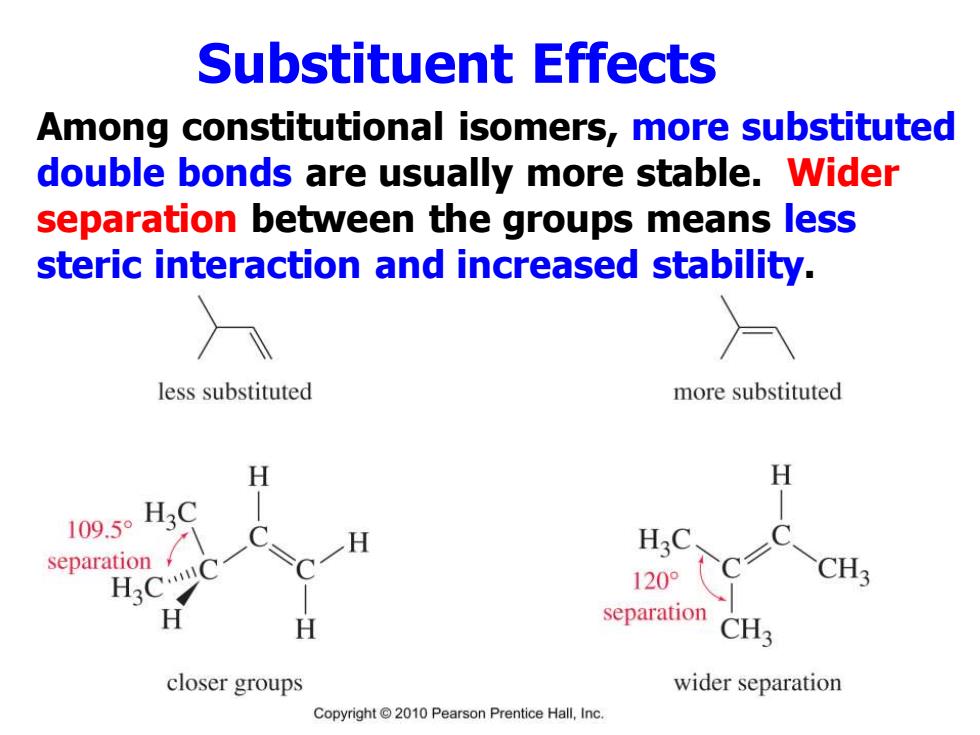
Substituent Effects Among constitutional isomers,more substituted double bonds are usually more stable.Wider separation between the groups means less steric interaction and increased stability. less substituted more substituted H H 109.50H3C separation 120° CH3 separation CH3 closer groups wider separation Copyright 2010 Pearson Prentice Hall,Inc
Substituent Effects Among constitutional isomers, more substituted double bonds are usually more stable. Wider separation between the groups means less steric interaction and increased stability