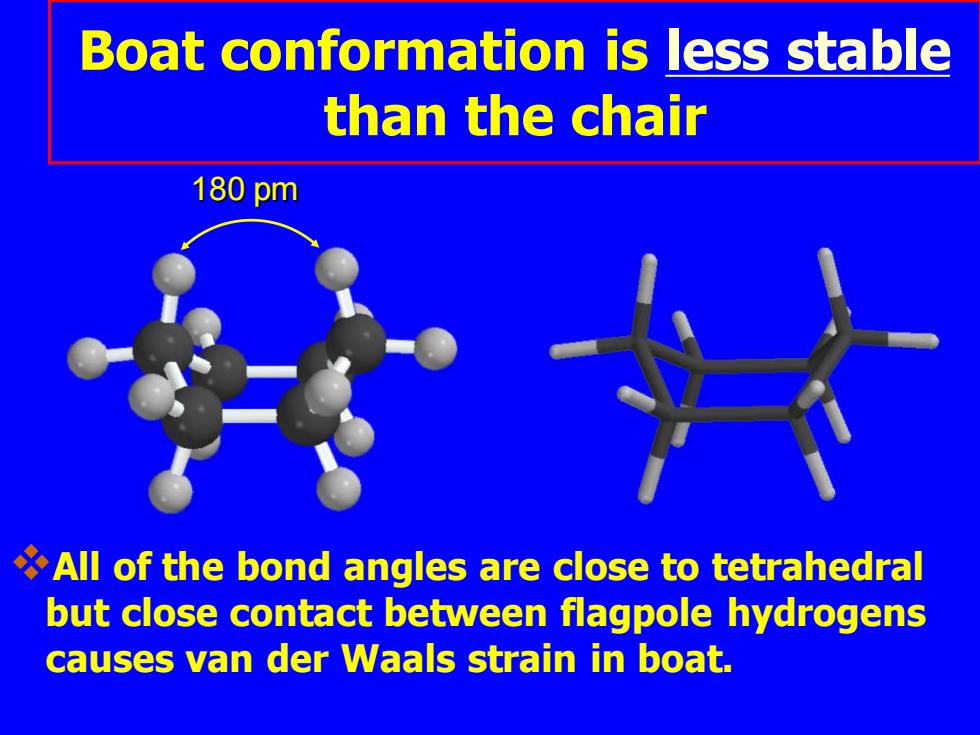
Boat conformation is less stable than the chair 180pm All of the bond angles are close to tetrahedral but close contact between flagpole hydrogens causes van der Waals strain in boat
❖All of the bond angles are close to tetrahedral but close contact between flagpole hydrogens causes van der Waals strain in boat. 180 pm Boat conformation is less stable than the chair
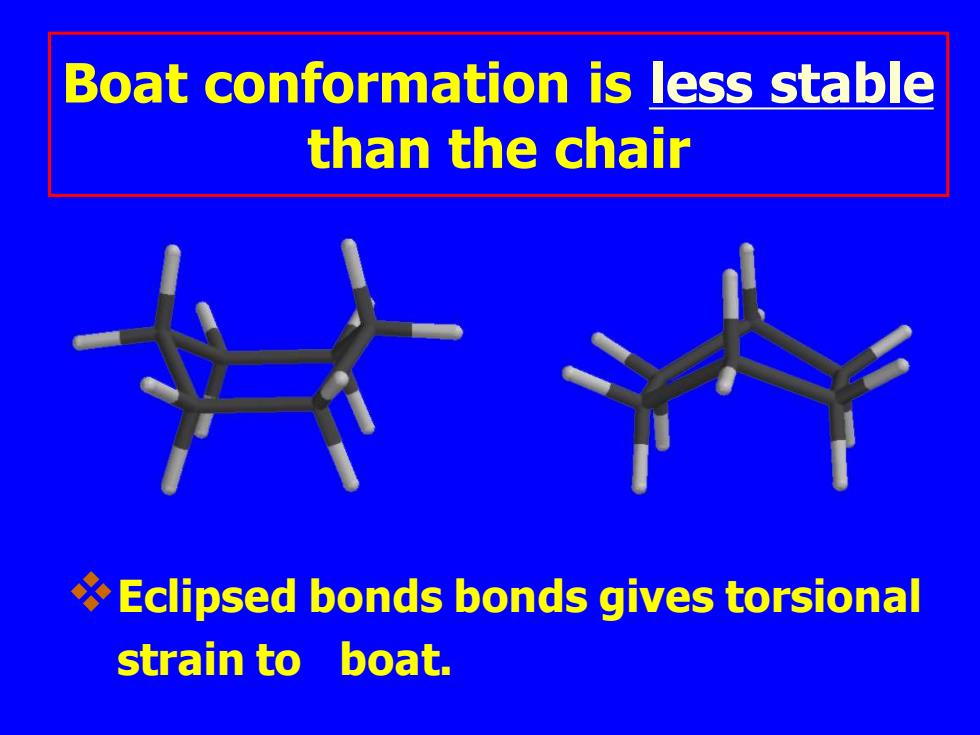
Boat conformation is less stable than the chair Eclipsed bonds bonds gives torsional strain to boat
❖Eclipsed bonds bonds gives torsional strain to boat. Boat conformation is less stable than the chair
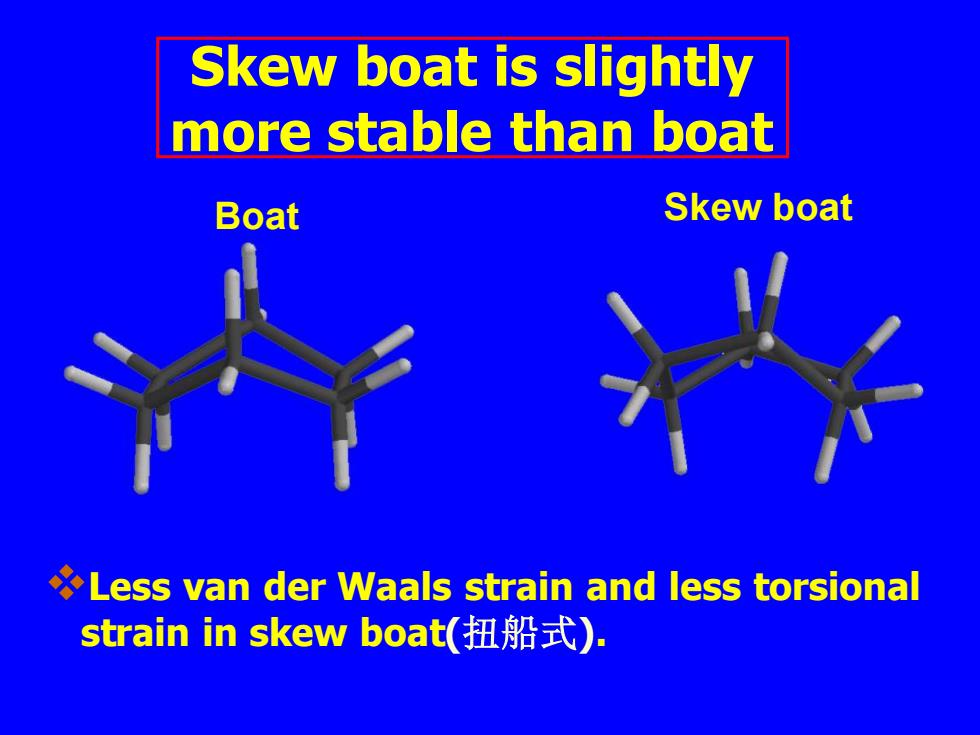
Skew boat is slightly more stable than boat Boat Skew boat Less van der Waals strain and less torsional strain in skew boat(扭船式)
❖Less van der Waals strain and less torsional strain in skew boat(扭船式). Boat Skew boat Skew boat is slightly more stable than boat

Generalization The chair conformation of cyclohexane is the most stable conformation and derivatives of cyclohexane almost always exist in the chair conformation 1 椅式构象最稳定 2) 取代基尽可能处于e键 3)较大基团处于e键
Generalization 1)椅式构象最稳定 2)取代基尽可能处于e键 3)较大基团处于e键 The chair conformation of cyclohexane is the most stable conformation and derivatives of cyclohexane almost always exist in the chair conformation
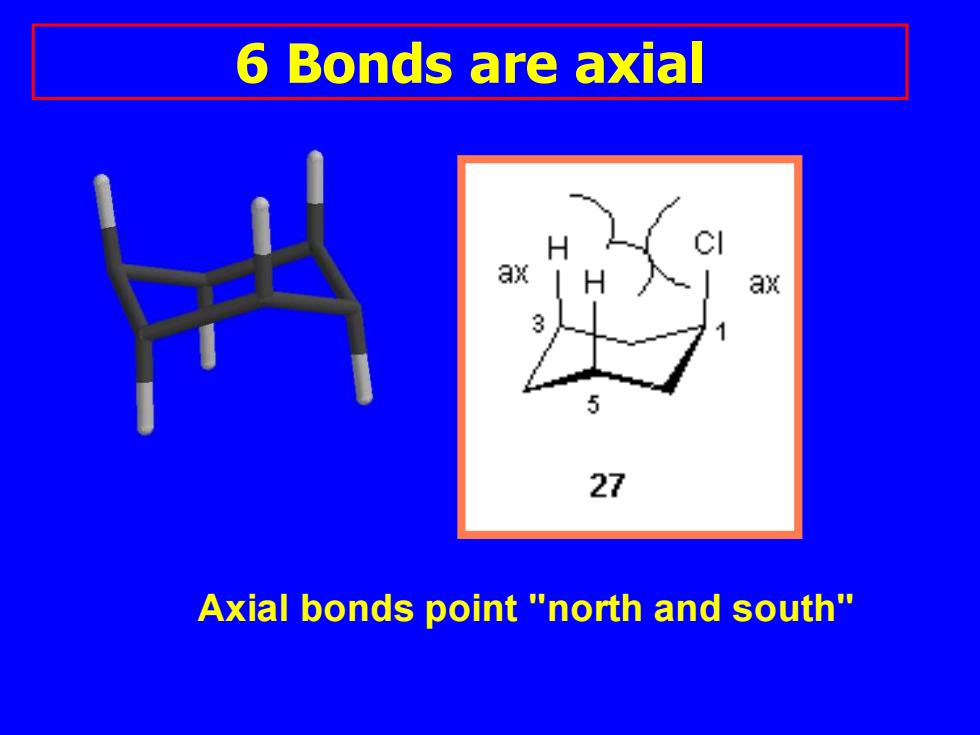
6 Bonds are axial a 27 Axial bonds point "north and south
Axial bonds point "north and south" 6 Bonds are axial